NPs Basic Information
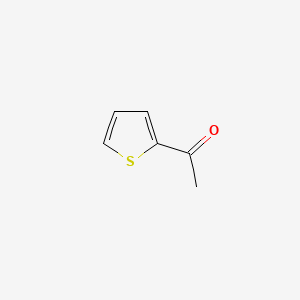
|
Name |
2-Acetylthiophene
|
| Molecular Formula | C6H6OS | |
| IUPAC Name* |
1-thiophen-2-ylethanone
|
|
| SMILES |
CC(=O)C1=CC=CS1
|
|
| InChI |
InChI=1S/C6H6OS/c1-5(7)6-3-2-4-8-6/h2-4H,1H3
|
|
| InChIKey |
WYJOVVXUZNRJQY-UHFFFAOYSA-N
|
|
| Synonyms |
2-ACETYLTHIOPHENE; 88-15-3; 1-thiophen-2-yl-ethanone; 1-(2-Thienyl)ethanone; 2-Acetothiophene; 2-Acetothienone; Methyl 2-thienyl ketone; Ethanone, 1-(2-thienyl)-; 1-(thiophen-2-yl)ethanone; 2-acetyl thiophene; 2-Thienyl methyl ketone; Ketone, methyl 2-thienyl; 1-thiophen-2-ylethanone; alpha-Acetylthiophene; 2-Acethylthiophene; 1-(2-thienyl)-ethanone; 1-(thiophen-2-yl)ethan-1-one; Methyl-2-thienyl ketone; 2-Acetylthiophen; NSC 2345; THIOPHENE,2-ACETYL; CHEMBL401911; 97511-16-5; 5ASO208T20; NSC-2345; 2-Aceto thiophene; EINECS 201-804-6; BRN 0107910; 2acetylthiophene; acetyl-thiophene; 2-acetylthiole; AI3-08491; 2-Acetyl-thiophene; MFCD00005442; ACETYLTHIOPHENE; 2-Acetylthiophene, 98%; SCHEMBL3798; WLN: T5SJ BV1; 2-(ACETYL)THIOFURAN; 5-17-09-00387 (Beilstein Handbook Reference); BIDD:GT0210; UNII-5ASO208T20; 1-(2-Thienyl)ethanone, 9CI; DTXSID2058960; NSC2345; CHEBI:179841; 2-Acetylthiophene, >=98%, FG; ZINC157402; BCP24442; STR01324; BDBM50376211; STL183822; 2-Acetylthiophene, analytical standard; AKOS000119817; AC-4918; AM82005; CS-W020047; PS-3261; DB-038374; 5-Acetyl-1-thia-1,3-cyclopentadiene-1-ium; A0724; FT-0610986; FT-0689587; EN300-17951; W18336; A842486; Q-100875; Q27261757; Z57127896; F0001-2186
|
|
| CAS | 88-15-3 | |
| PubChem CID | 6920 | |
| ChEMBL ID | CHEMBL401911 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 126.18 | ALogp: | 1.2 |
| HBD: | 0 | HBA: | 2 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 45.3 | Aromatic Rings: | 1 |
| Heavy Atoms: | 8 | QED Weighted: | 0.528 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.056 | MDCK Permeability: | 0.00007650 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.006 | 20% Bioavailability (F20%): | 0.017 |
| 30% Bioavailability (F30%): | 0.278 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.402 | Plasma Protein Binding (PPB): | 79.96% |
| Volume Distribution (VD): | 0.616 | Fu: | 33.92% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.973 | CYP1A2-substrate: | 0.906 |
| CYP2C19-inhibitor: | 0.813 | CYP2C19-substrate: | 0.386 |
| CYP2C9-inhibitor: | 0.189 | CYP2C9-substrate: | 0.661 |
| CYP2D6-inhibitor: | 0.687 | CYP2D6-substrate: | 0.581 |
| CYP3A4-inhibitor: | 0.031 | CYP3A4-substrate: | 0.306 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 5.195 | Half-life (T1/2): | 0.562 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.035 | Human Hepatotoxicity (H-HT): | 0.036 |
| Drug-inuced Liver Injury (DILI): | 0.676 | AMES Toxicity: | 0.367 |
| Rat Oral Acute Toxicity: | 0.16 | Maximum Recommended Daily Dose: | 0.025 |
| Skin Sensitization: | 0.361 | Carcinogencity: | 0.068 |
| Eye Corrosion: | 0.916 | Eye Irritation: | 0.994 |
| Respiratory Toxicity: | 0.979 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001141 | 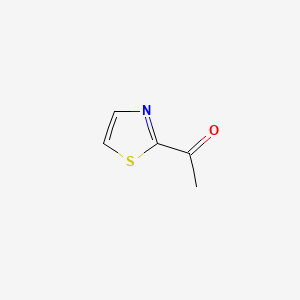 |
0.375 | D07BPS | 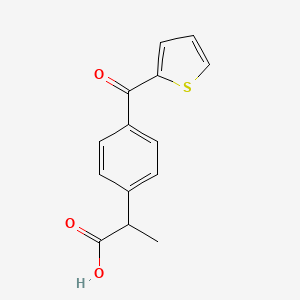 |
0.333 | ||
| ENC000480 | 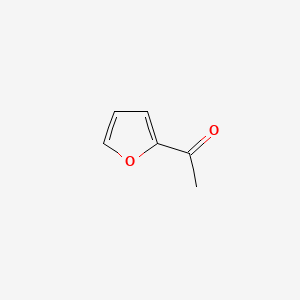 |
0.375 | D0GY5Z | 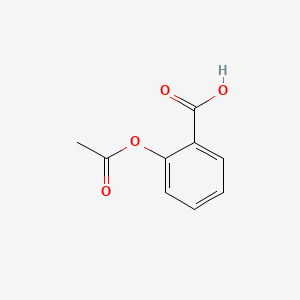 |
0.239 | ||
| ENC000640 | 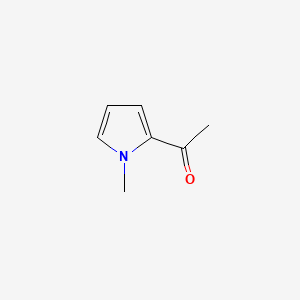 |
0.353 | D0U5QK | 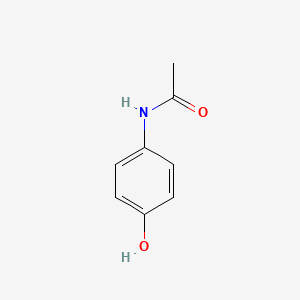 |
0.238 | ||
| ENC000192 | 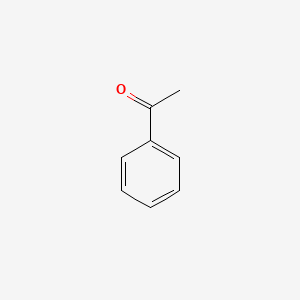 |
0.343 | D0GA5T |  |
0.238 | ||
| ENC000690 | 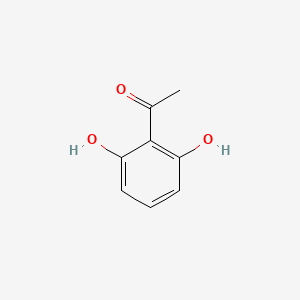 |
0.308 | D06NVJ | 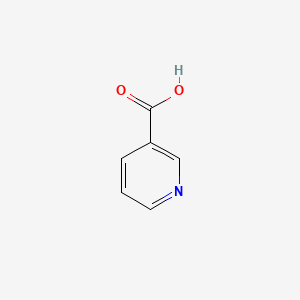 |
0.237 | ||
| ENC000612 | 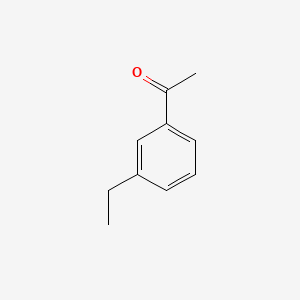 |
0.300 | D0X9RY |  |
0.237 | ||
| ENC000200 | 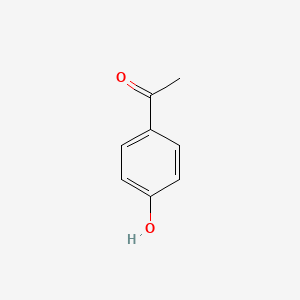 |
0.289 | D07HBX |  |
0.225 | ||
| ENC000218 | 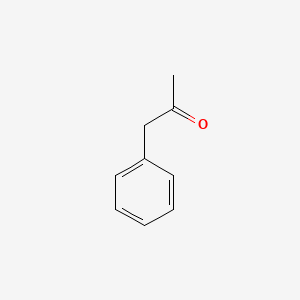 |
0.282 | D0O2WB |  |
0.213 | ||
| ENC001108 | 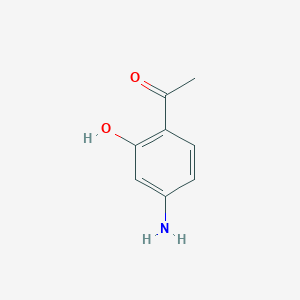 |
0.275 | D01PLN | 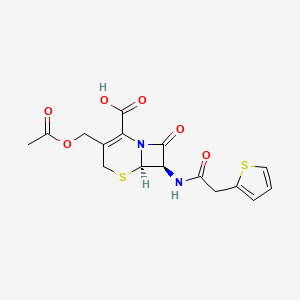 |
0.205 | ||
| ENC000344 | 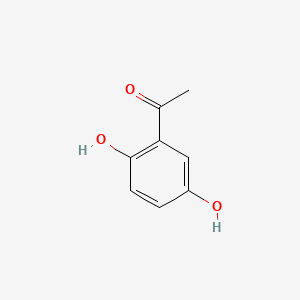 |
0.275 | D01ZJK |  |
0.205 | ||