NPs Basic Information
|
Name |
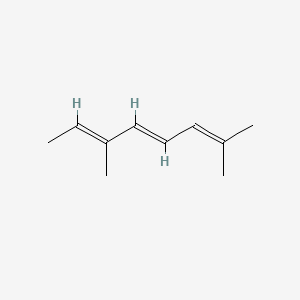
2,6-Dimethyl-2,4,6-octatriene
|
| Molecular Formula | C10H16 | |
| IUPAC Name* |
(4E,6E)-2,6-dimethylocta-2,4,6-triene
|
|
| SMILES |
C/C=C(\C)/C=C/C=C(C)C
|
|
| InChI |
InChI=1S/C10H16/c1-5-10(4)8-6-7-9(2)3/h5-8H,1-4H3/b8-6+,10-5+
|
|
| InChIKey |
GQVMHMFBVWSSPF-SOYUKNQTSA-N
|
|
| Synonyms |
2,6-Dimethyl-2,4,6-octatriene; Alloocimene; 673-84-7; ALLO-OCIMENE; 3016-19-1; (4E,6E)-Alloocimene; trans,trans-Alloocimene; (4E,6E)-Allocimene; (4E,6E)-2,6-dimethylocta-2,4,6-triene; 2,6-Dimethylocta-2,4,6-triene; (E,E)-2,6-Dimethyl-2,4,6-octatriene; 2,4,6-Octatriene, 2,6-dimethyl-, (4E,6E)-; 2,4,6-Octatriene, 2,6-dimethyl-; 2,4,6-Octatriene, 2,6-dimethyl-, (E,E)-; trans-Alloocimene; (E,E)-2,6-Dimethylocta-2,4,6-triene; Alloocimene, (4E,6E)-; trans-allo-ocimene; 6TF53L340E; 2,6-Dimethyl-2,4E,6E-octatriene; DSSTox_CID_7288; DSSTox_RID_78388; DSSTox_GSID_27288; Allocymene; OCIMENE, ALLO; CAS-673-84-7; EINECS 211-614-5; EINECS 221-153-1; NSC 406263; Neoalloocimene; UNII-6TF53L340E; AI3-00737; Alloocimene I; NSC406263; cis-Allo-ocimene; ocimene (allo-); Z-Neo-allo-ocimene; (E,E)-allo-ocimene; ALLOOCIMENE A; trans,trans-allo-ocimene; Alloocimene, tech. grade; UNII-J9D0BS5BZN; (4E,6E)-allo-ocimene; J9D0BS5BZN; (E,E)-ALLOOCIMENE; OCIMENE, A110; 4-trans-6-trans-alloocimene; ALLOCYMENE APPROX.80%; CHEMBL2268552; CHEBI:90064; DTXSID00883936; CHEBI:141222; ZINC1599076; Tox21_202278; Tox21_303244; BBL027750; LMFA11000042; MFCD00009278; STL146333; AKOS005720971; NSC-406263; NCGC00249203-01; NCGC00257015-01; NCGC00259827-01; (2E)-3,7-Dimethyl-2,4,6-octatriene; (2Z)-3,7-Dimethyl-2,4,6-octatriene; VS-08587; 2,6-Dimethyl-octa-2,4,6-triene, trans; 2,6-Octatriene, 2,6-dimethyl- (VAN8C; 2,6-dimethyl-octa-2,4trans,6trans-triene; (4E,6E)-2,6-Dimethyl-2,4,6-octatriene; 2,4,6-Octatriene, 2,6-dimethyl- (VAN); D1277; 2,6-Dimethyl-2,4,6-octatriene, mixture of isomers; W-109590; W-109849; Q15628365; 2,6-Dimethyl-2,4,6-octatriene, technical grade, 80%
|
|
| CAS | 3016-19-1 | |
| PubChem CID | 5368821 | |
| ChEMBL ID | CHEMBL2268552 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 136.23 | ALogp: | 4.2 |
| HBD: | 0 | HBA: | 0 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 0.0 | Aromatic Rings: | 0 |
| Heavy Atoms: | 10 | QED Weighted: | 0.499 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.188 | MDCK Permeability: | 0.00001990 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.006 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.003 |
| 30% Bioavailability (F30%): | 0.01 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.838 | Plasma Protein Binding (PPB): | 89.10% |
| Volume Distribution (VD): | 1.624 | Fu: | 18.23% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.833 | CYP1A2-substrate: | 0.959 |
| CYP2C19-inhibitor: | 0.559 | CYP2C19-substrate: | 0.942 |
| CYP2C9-inhibitor: | 0.121 | CYP2C9-substrate: | 0.975 |
| CYP2D6-inhibitor: | 0.785 | CYP2D6-substrate: | 0.932 |
| CYP3A4-inhibitor: | 0.03 | CYP3A4-substrate: | 0.404 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 5.741 | Half-life (T1/2): | 0.734 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.013 | Human Hepatotoxicity (H-HT): | 0.068 |
| Drug-inuced Liver Injury (DILI): | 0.042 | AMES Toxicity: | 0.009 |
| Rat Oral Acute Toxicity: | 0.013 | Maximum Recommended Daily Dose: | 0.705 |
| Skin Sensitization: | 0.922 | Carcinogencity: | 0.392 |
| Eye Corrosion: | 0.978 | Eye Irritation: | 0.995 |
| Respiratory Toxicity: | 0.279 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001740 | 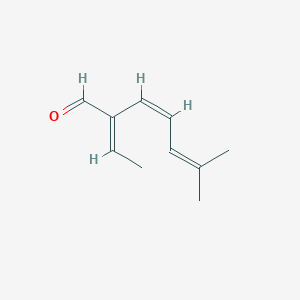 |
0.571 | D0S7WX | 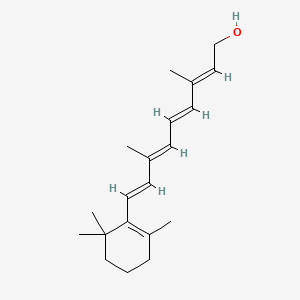 |
0.277 | ||
| ENC001718 | 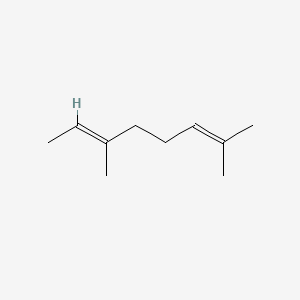 |
0.368 | D0G3PI | 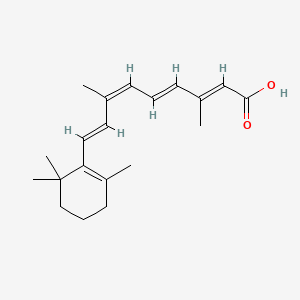 |
0.269 | ||
| ENC001568 | 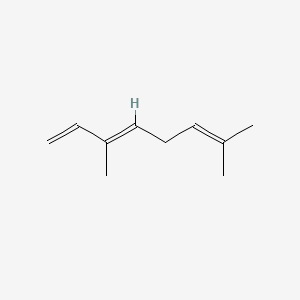 |
0.333 | D02DGU | 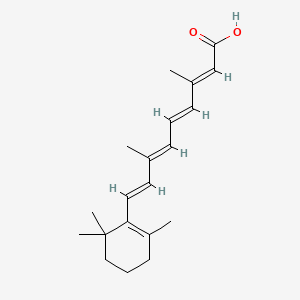 |
0.269 | ||
| ENC000526 | 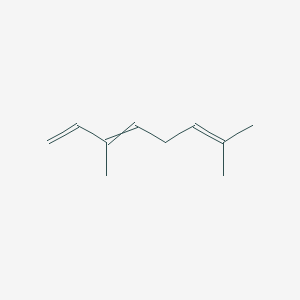 |
0.333 | D00DKK | 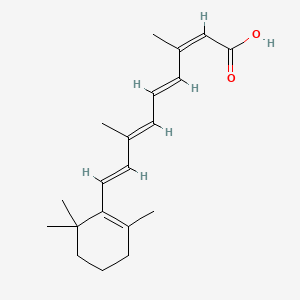 |
0.269 | ||
| ENC001629 | 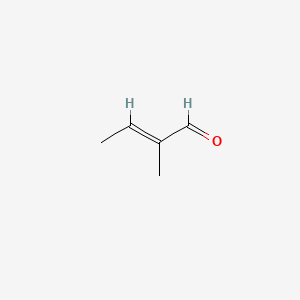 |
0.323 | D05QDC | 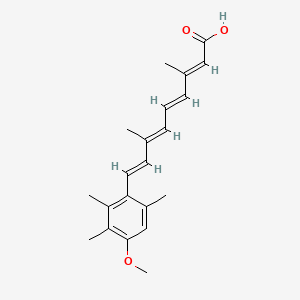 |
0.250 | ||
| ENC003853 | 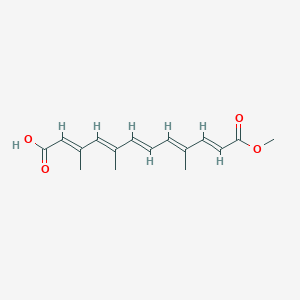 |
0.317 | D0B1IP |  |
0.231 | ||
| ENC003854 |  |
0.317 | D0M1PQ |  |
0.209 | ||
| ENC003852 |  |
0.310 | D05XQE | 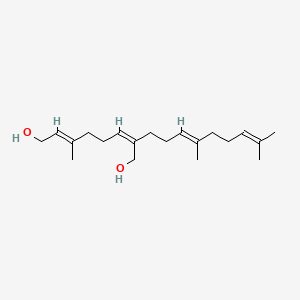 |
0.194 | ||
| ENC001424 | 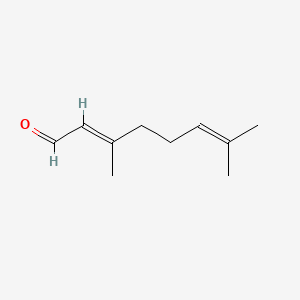 |
0.310 | D09XWD | 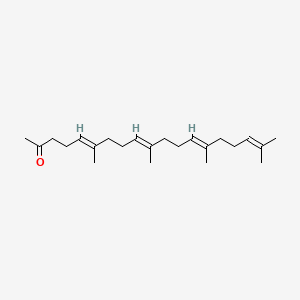 |
0.182 | ||
| ENC001434 | 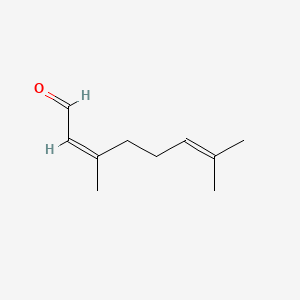 |
0.310 | D0MY8N |  |
0.162 | ||