NPs Basic Information
|
Name |
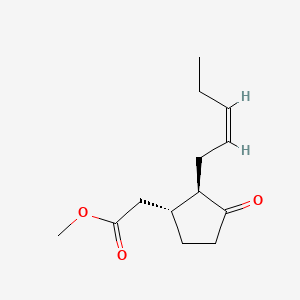
Methyl jasmonate
|
| Molecular Formula | C13H20O3 | |
| IUPAC Name* |
methyl 2-[(1R,2R)-3-oxo-2-[(Z)-pent-2-enyl]cyclopentyl]acetate
|
|
| SMILES |
CC/C=C\C[C@@H]1[C@H](CCC1=O)CC(=O)OC
|
|
| InChI |
InChI=1S/C13H20O3/c1-3-4-5-6-11-10(7-8-12(11)14)9-13(15)16-2/h4-5,10-11H,3,6-9H2,1-2H3/b5-4-/t10-,11-/m1/s1
|
|
| InChIKey |
GEWDNTWNSAZUDX-WQMVXFAESA-N
|
|
| Synonyms |
Methyl jasmonate; 1211-29-6; (-)-Methyl jasmonate; Methyl cis-jasmonate; Methyl 2-((1R,2R)-3-oxo-2-((Z)-pent-2-en-1-yl)cyclopentyl)acetate; methyl (-)-jasmonate; Jasmonic acid methyl ester; (-)-Jasmonic acid methyl ester; (3R,7R)-Methyl jasmonate; Methyl jasmonic acid; CHEBI:15929; Methyl (2-pent-2-enyl-3-oxo-1-cyclopentyl)acetate; 3-oxo-2-(2-pentenyl)cyclopentaneacetic acid methyl ester; methyl 2-[(1R,2R)-3-oxo-2-[(Z)-pent-2-enyl]cyclopentyl]acetate; Z-Methyl jasmonoate; Cyclopentaneacetic acid, 3-oxo-2-(2Z)-2-pentenyl-, methyl ester, (1R,2R)-; 900N171A0F; (-)-Jasmonic acid, methyl ester (trans); Cyclopentaneacetic acid, 3-oxo-2-(2-pentenyl)-, methyl ester, (Z)-trans-; Methyl 3-oxo-2-(2-pentenyl)cyclopentaneacetate, (1R-(1alpha,2beta(Z)))-; Cyclopentaneacetic acid, 3-oxo-2-(2Z)-2-penten-1-yl-, methyl ester, (1R,2R)-; Methyljasmonate; methyl 2-((1R,2R)-3-oxo-2-pent-2Z-enyl)cyclopentyl)acetate; Methyl (1R-(1alpha,2beta(Z)))-3-oxo-2-(pent-2-enyl)cyclopentaneacetate; Cyclopentaneacetic acid, 3-oxo-2-(2-pentenyl)-, methyl ester, (1R-(1alpha,2beta(Z)))-; Cyclopentaneacetic acid, 3-oxo-2-(2-pentenyl)-, methyl ester, (1theta-(1alpha,2beta(Z)))-; Methyl dl-jasmonate; Cyclopentaneacetic acid, 3-oxo-trans-2-(cis-2-pentenyl), methyl ester; Methyl 3-oxo-2-(2-pentenyl)cyclopentaneacetate; 39924-52-2; FEMA No. 3410; UNII-900N171A0F; HSDB 8131; Cyclopentaneacetic acid, 3-oxo-2-(2-pentenyl)-, methyl ester, [1R-[1alpha,2beta(Z)]]-; Cyclopentaneacetic acid, 3-oxo-2-(2Z)-2-pentenyl-, methyl ester, (1R,2R)-rel-; 20073-13-6; CYCLOPENTANEACETIC ACID, 3-OXO-2-(2-PENTENYL)-, METHYL ESTER, (1R-(1.ALPHA.,2.BETA.(Z)))-; EINECS 214-918-6; EINECS 243-497-1; SCHEMBL36186; METHYL JASMONATE [FHFI]; CHEMBL2139332; DTXSID3036731; FEMA 3410; (3R,7R)-(?)-Methyl jasmonate; Methyl 3-oxo-2-(2-pentenyl)cyclopentaneacetate, (Z)-trans-; CMC_7389; ZINC4654657; BDBM50509748; CMC_13964; LMFA02020010; (+-)-Cyclopentaneacetic acid, 3-oxo-trans-2-(cis-2-pentenyl), methyl ester; AKOS015950850; JASMONIC ACID METHYL ESTER [MI]; AS-75742; HY-135663; CS-0113712; D94878; A891945; Q26840883; 8171C3E0-B789-4211-B5C1-88C6B260AE3C; Cyclopentaneaceticacid,3-oxo-2-(2Z)-2-penten-1-yl-,methyl ester,(1R,2R)-; Methyl (1alpha,2beta(Z))-(1)-3-oxo-2-(pent-2-enyl)cyclopentaneacetate; methyl {(1R,2R)-3-oxo-2-[(2Z)-pent-2-en-1-yl]cyclopentyl}acetate; Cyclopentaneacetic acid, 3-oxo-2-(2-pentenyl)-, methyl ester, (Z)-trans- (8CI); Cyclopentaneacetic acid, 3-oxo-2-(2Z)-2-penten-1-yl-, methyl ester, (1R,2R)-rel-; Cyclopentaneacetic acid, 3-oxo-2-(2Z)-2-pentenyl-, methyl ester, (1R,2R)- (9CI); Cyclopentaneacetic acid, 3-oxo-2-[(2Z)-2-pentenyl]-, methyl ester, (1R,2R)-; Cyclopentaneaceticacid, 3-oxo-2-(2Z)-2-penten-1-yl-, methyl ester, (1R,2R)-; 139442-00-5
|
|
| CAS | 1211-29-6 | |
| PubChem CID | 5281929 | |
| ChEMBL ID | CHEMBL2139332 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 224.3 | ALogp: | 1.9 |
| HBD: | 0 | HBA: | 3 |
| Rotatable Bonds: | 6 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 43.4 | Aromatic Rings: | 1 |
| Heavy Atoms: | 16 | QED Weighted: | 0.531 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.576 | MDCK Permeability: | 0.00004840 |
| Pgp-inhibitor: | 0.059 | Pgp-substrate: | 0.003 |
| Human Intestinal Absorption (HIA): | 0.013 | 20% Bioavailability (F20%): | 0.899 |
| 30% Bioavailability (F30%): | 0.975 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.966 | Plasma Protein Binding (PPB): | 66.70% |
| Volume Distribution (VD): | 0.36 | Fu: | 34.84% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.091 | CYP1A2-substrate: | 0.553 |
| CYP2C19-inhibitor: | 0.211 | CYP2C19-substrate: | 0.858 |
| CYP2C9-inhibitor: | 0.105 | CYP2C9-substrate: | 0.636 |
| CYP2D6-inhibitor: | 0.006 | CYP2D6-substrate: | 0.859 |
| CYP3A4-inhibitor: | 0.133 | CYP3A4-substrate: | 0.323 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 9.458 | Half-life (T1/2): | 0.941 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.011 | Human Hepatotoxicity (H-HT): | 0.425 |
| Drug-inuced Liver Injury (DILI): | 0.258 | AMES Toxicity: | 0.027 |
| Rat Oral Acute Toxicity: | 0.139 | Maximum Recommended Daily Dose: | 0.724 |
| Skin Sensitization: | 0.137 | Carcinogencity: | 0.799 |
| Eye Corrosion: | 0.176 | Eye Irritation: | 0.388 |
| Respiratory Toxicity: | 0.07 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC005598 | 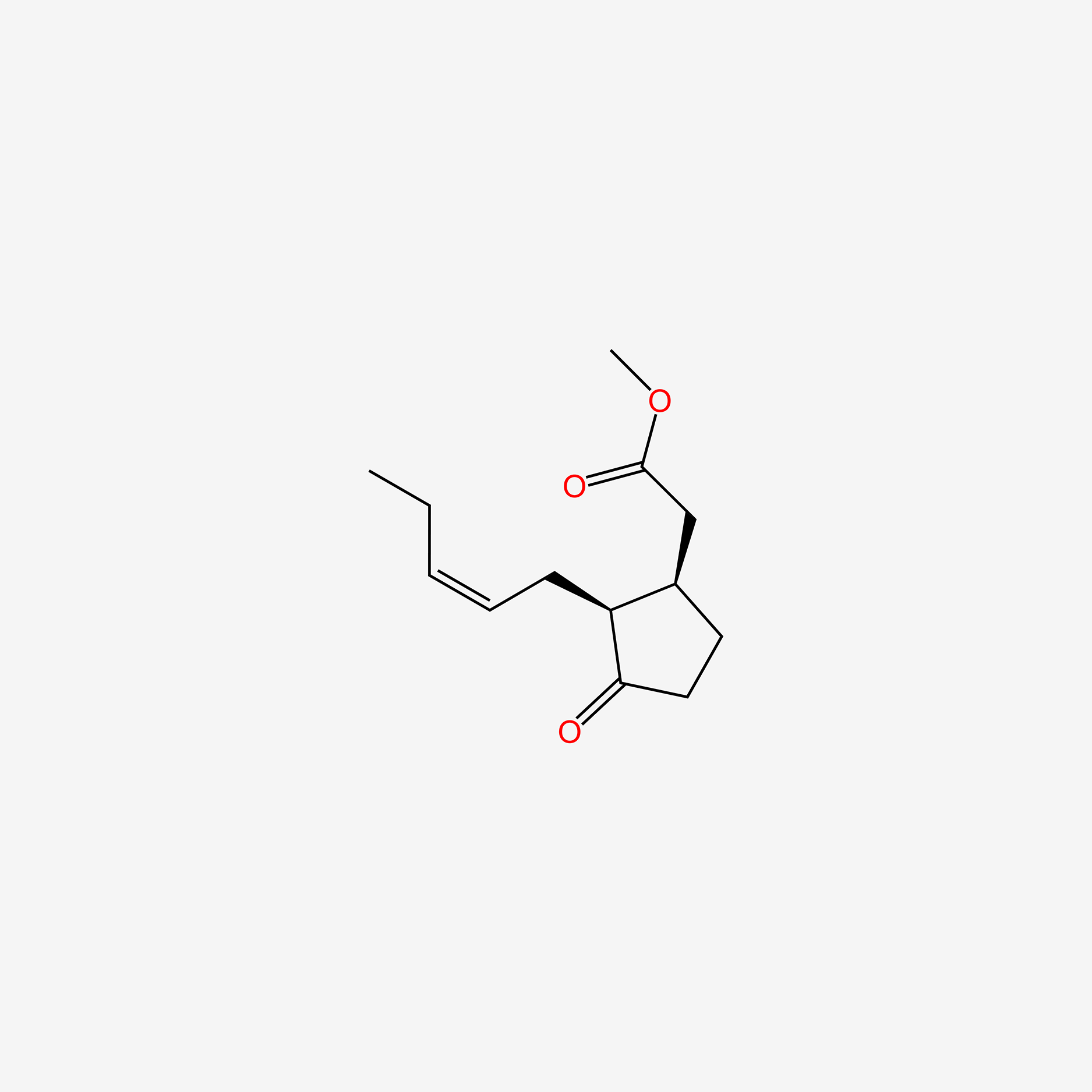 |
1.000 | D09ANG | 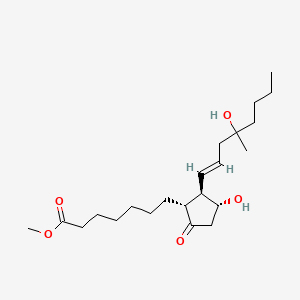 |
0.269 | ||
| ENC001659 | 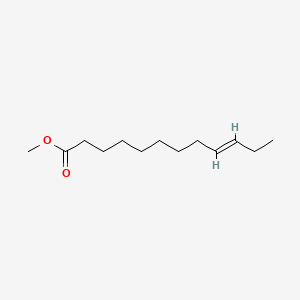 |
0.323 | D06FEA | 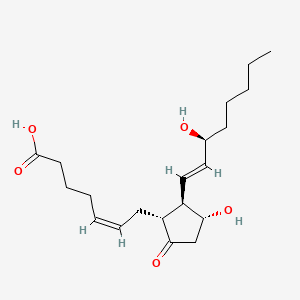 |
0.256 | ||
| ENC001642 | 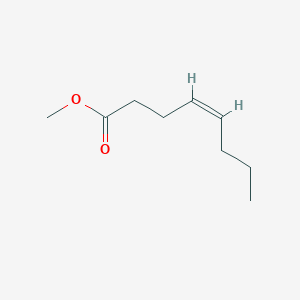 |
0.321 | D0ZI4H | 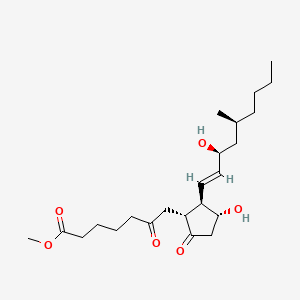 |
0.255 | ||
| ENC001840 | 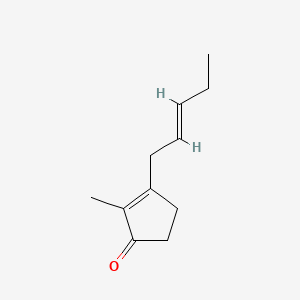 |
0.305 | D0OL6O | 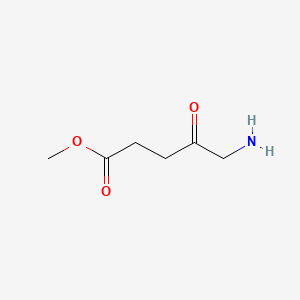 |
0.228 | ||
| ENC001459 | 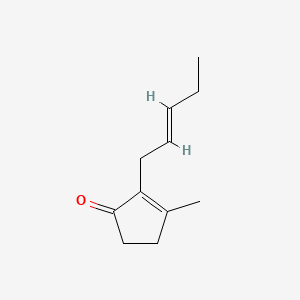 |
0.305 | D0QQ6Q | 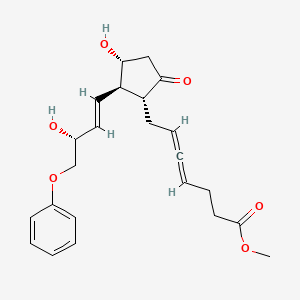 |
0.223 | ||
| ENC003364 | 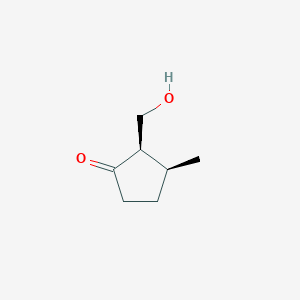 |
0.283 | D0CT4D | 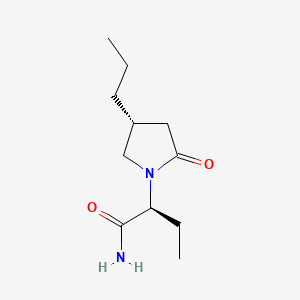 |
0.217 | ||
| ENC004359 | 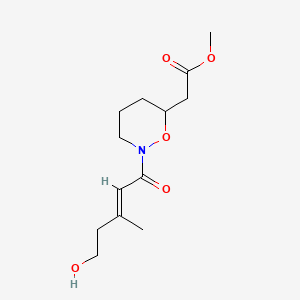 |
0.280 | D0X2UE | 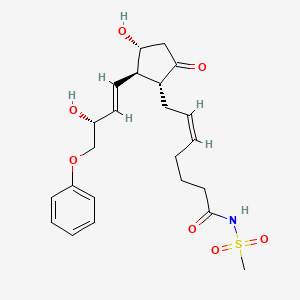 |
0.209 | ||
| ENC005384 | 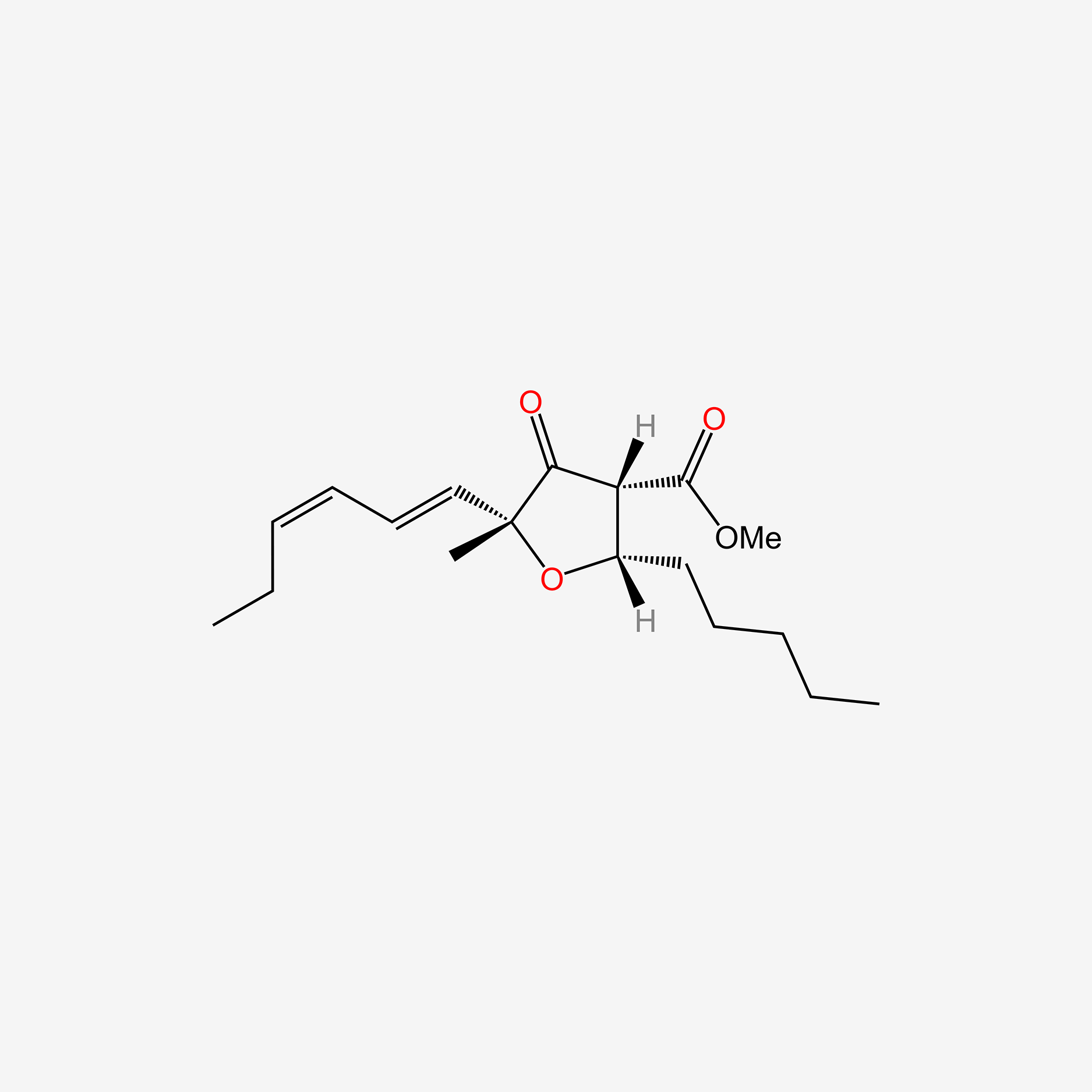 |
0.268 | D0N3NO |  |
0.204 | ||
| ENC001696 |  |
0.262 | D0H2YX |  |
0.198 | ||
| ENC000235 |  |
0.259 | D0G2MW |  |
0.189 | ||