NPs Basic Information
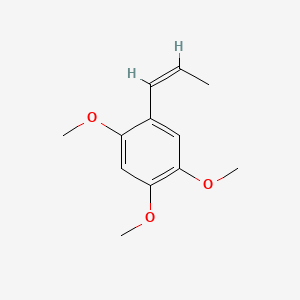
|
Name |
beta-Asarone
|
| Molecular Formula | C12H16O3 | |
| IUPAC Name* |
1,2,4-trimethoxy-5-[(Z)-prop-1-enyl]benzene
|
|
| SMILES |
C/C=C\C1=CC(=C(C=C1OC)OC)OC
|
|
| InChI |
InChI=1S/C12H16O3/c1-5-6-9-7-11(14-3)12(15-4)8-10(9)13-2/h5-8H,1-4H3/b6-5-
|
|
| InChIKey |
RKFAZBXYICVSKP-WAYWQWQTSA-N
|
|
| Synonyms |
beta-Asarone; 5273-86-9; cis-Isoelemicin; cis-Isoasarone; (Z)-Asarone; cis-Asarone; cis-2,4,5-Trimethoxy-1-propenylbenzene; Cis-Asaron; .beta.-Asarone; cis-.beta.-Asarone; (Z)-5-Propenyl-1,2,4-trimethoxybenzene; IGA3MH6IUW; (Z)-1,2,4-Trimethoxy-5-(1-propenyl)benzene; 2,4,5-Trimethoxypropen-1-ylbenzene; 1,2,4-trimethoxy-5-[(Z)-prop-1-enyl]benzene; BENZENE, 1,2,4-TRIMETHOXY-5-PROPENYL-, (Z)-; CHEBI:10353; Benzene, 1,2,4-trimethoxy-5-(1-propenyl)-, (Z)-; cis-1-Propenyl-2,4,5-trimethoxybenzene; UNII-IGA3MH6IUW; beta-asaron; CCRIS 1592; EINECS 226-096-6; I(2)-Asarone; BRN 1910605; 1,2,4-Trimethoxy-5-((Z)-1-propenyl)benzene; 1,2,4-trimethoxy-5-(prop-1-en-1-yl)benzene; (Z)-.beta.-Asarone; AI3-36897; 3-06-00-06440 (Beilstein Handbook Reference); SCHEMBL528747; .BETA.-ASARONE, CIS-; CHEMBL477752; CHEBI:68146; CHEBI:78308; cis-2,4,5-trimethoxyphenylpropene; DTXSID601020057; HMS3886B18; BCP23722; HY-N1501; MFCD00009281; s9118; ZINC13424754; AKOS030524038; CCG-266642; CS-W009103; DS-9662; 1,2,4-trimethoxy-5-propenylbenzene, z-; AC-34897; (Z)-1-(2,4,5-Trimethoxyphenyl)-1-propene; cis-2,4,5-Trimethoxy-1-propenylbenzene, 70%; 1,2,4-Trimethoxy-5-[(1Z)-1-propenyl]benzene; A870918; W-105801; 1,2,4-trimethoxy-5-[(1Z)-prop-1-en-1-yl]benzene; beta-Asarone, primary pharmaceutical reference standard; Q27089385; 1,2,4-Trimethoxy-5-((z)-1-propenyl)benzene, ?-asarone; (z)-1 pound not2 pound not4-trimethoxy-5-(1-propenyl)benzene; BENZENE, 1,2,4-TRIMETHOXY-5-(1Z)-1-PROPEN-1-YL-
|
|
| CAS | 5273-86-9 | |
| PubChem CID | 5281758 | |
| ChEMBL ID | CHEMBL477752 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 208.25 | ALogp: | 3.0 |
| HBD: | 0 | HBA: | 3 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 27.7 | Aromatic Rings: | 1 |
| Heavy Atoms: | 15 | QED Weighted: | 0.757 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.427 | MDCK Permeability: | 0.00002530 |
| Pgp-inhibitor: | 0.038 | Pgp-substrate: | 0.856 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.029 |
| 30% Bioavailability (F30%): | 0.313 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.933 | Plasma Protein Binding (PPB): | 93.36% |
| Volume Distribution (VD): | 1.325 | Fu: | 7.38% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.951 | CYP1A2-substrate: | 0.964 |
| CYP2C19-inhibitor: | 0.258 | CYP2C19-substrate: | 0.919 |
| CYP2C9-inhibitor: | 0.031 | CYP2C9-substrate: | 0.636 |
| CYP2D6-inhibitor: | 0.029 | CYP2D6-substrate: | 0.881 |
| CYP3A4-inhibitor: | 0.09 | CYP3A4-substrate: | 0.613 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 11.093 | Half-life (T1/2): | 0.818 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.019 | Human Hepatotoxicity (H-HT): | 0.293 |
| Drug-inuced Liver Injury (DILI): | 0.765 | AMES Toxicity: | 0.138 |
| Rat Oral Acute Toxicity: | 0.039 | Maximum Recommended Daily Dose: | 0.039 |
| Skin Sensitization: | 0.162 | Carcinogencity: | 0.142 |
| Eye Corrosion: | 0.116 | Eye Irritation: | 0.888 |
| Respiratory Toxicity: | 0.045 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001410 | 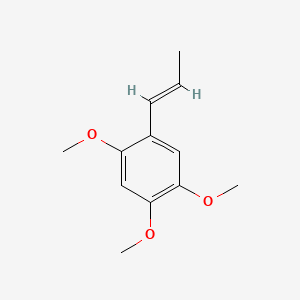 |
1.000 | D0NJ3V | 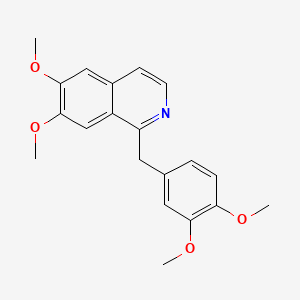 |
0.349 | ||
| ENC001461 |  |
0.510 | D01FFA | 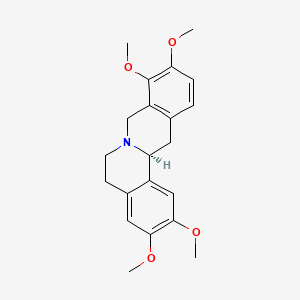 |
0.337 | ||
| ENC000340 | 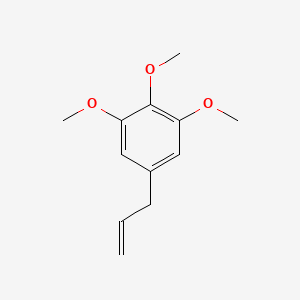 |
0.439 | D0AO5H | 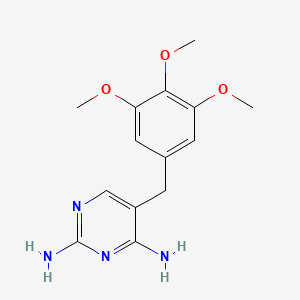 |
0.320 | ||
| ENC005700 | 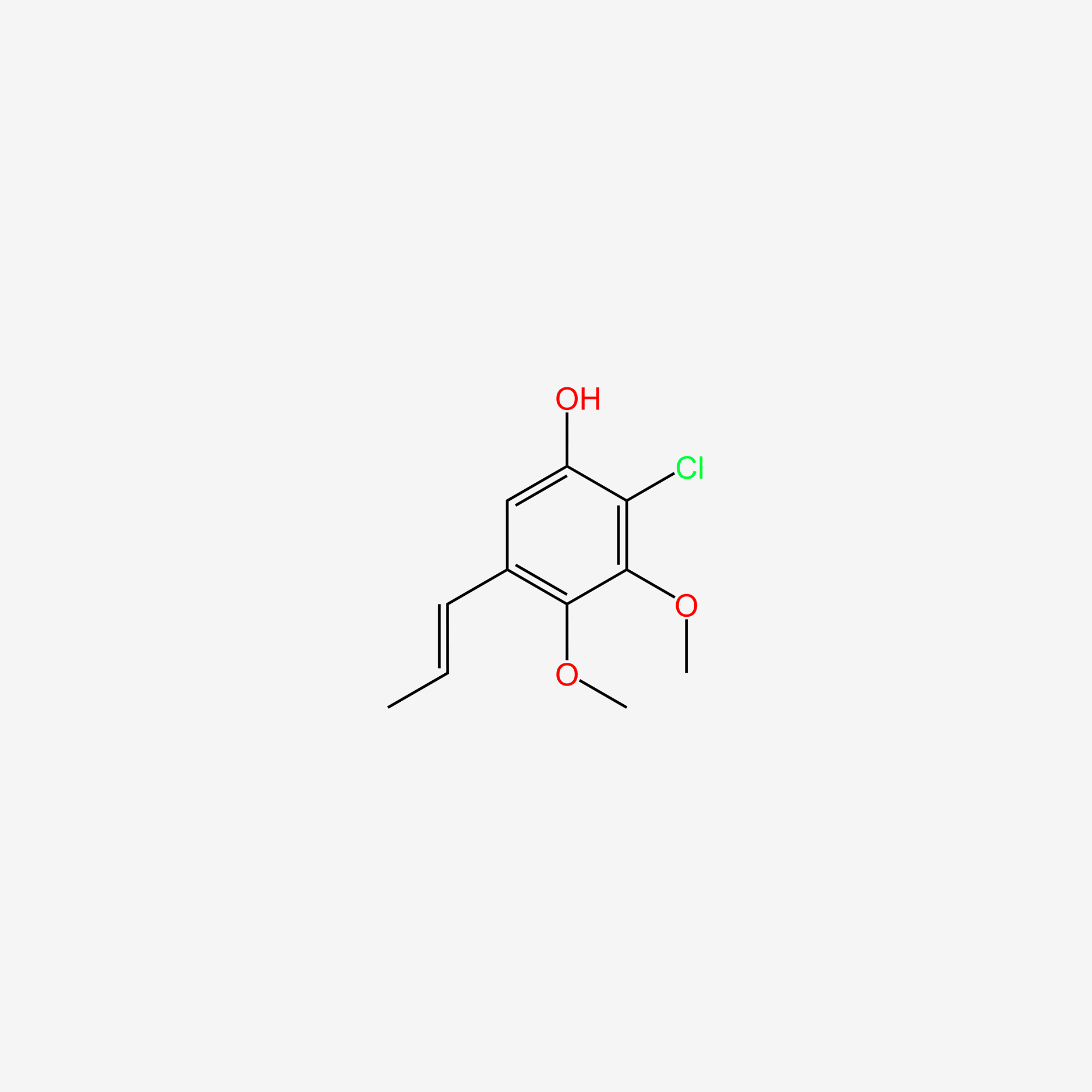 |
0.397 | D0C1SF | 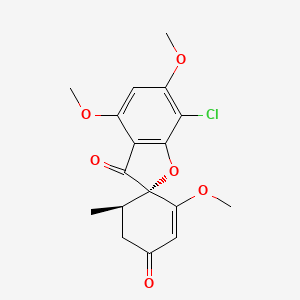 |
0.309 | ||
| ENC000304 | 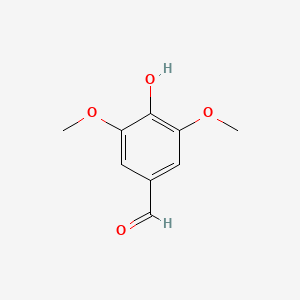 |
0.382 | D09PJX | 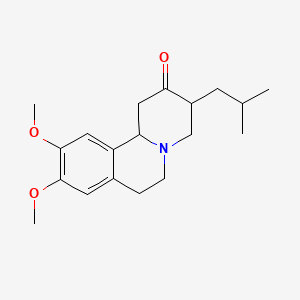 |
0.296 | ||
| ENC001376 | 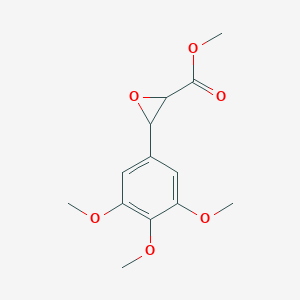 |
0.368 | D06GCK |  |
0.294 | ||
| ENC000501 | 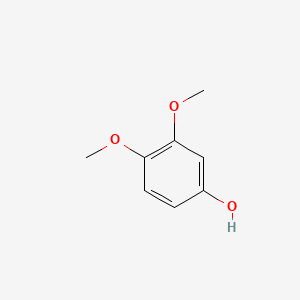 |
0.365 | D0R0FE | 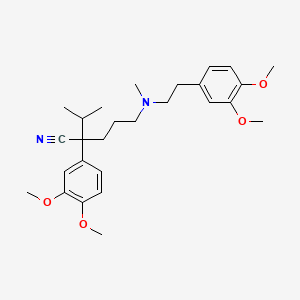 |
0.282 | ||
| ENC005938 | 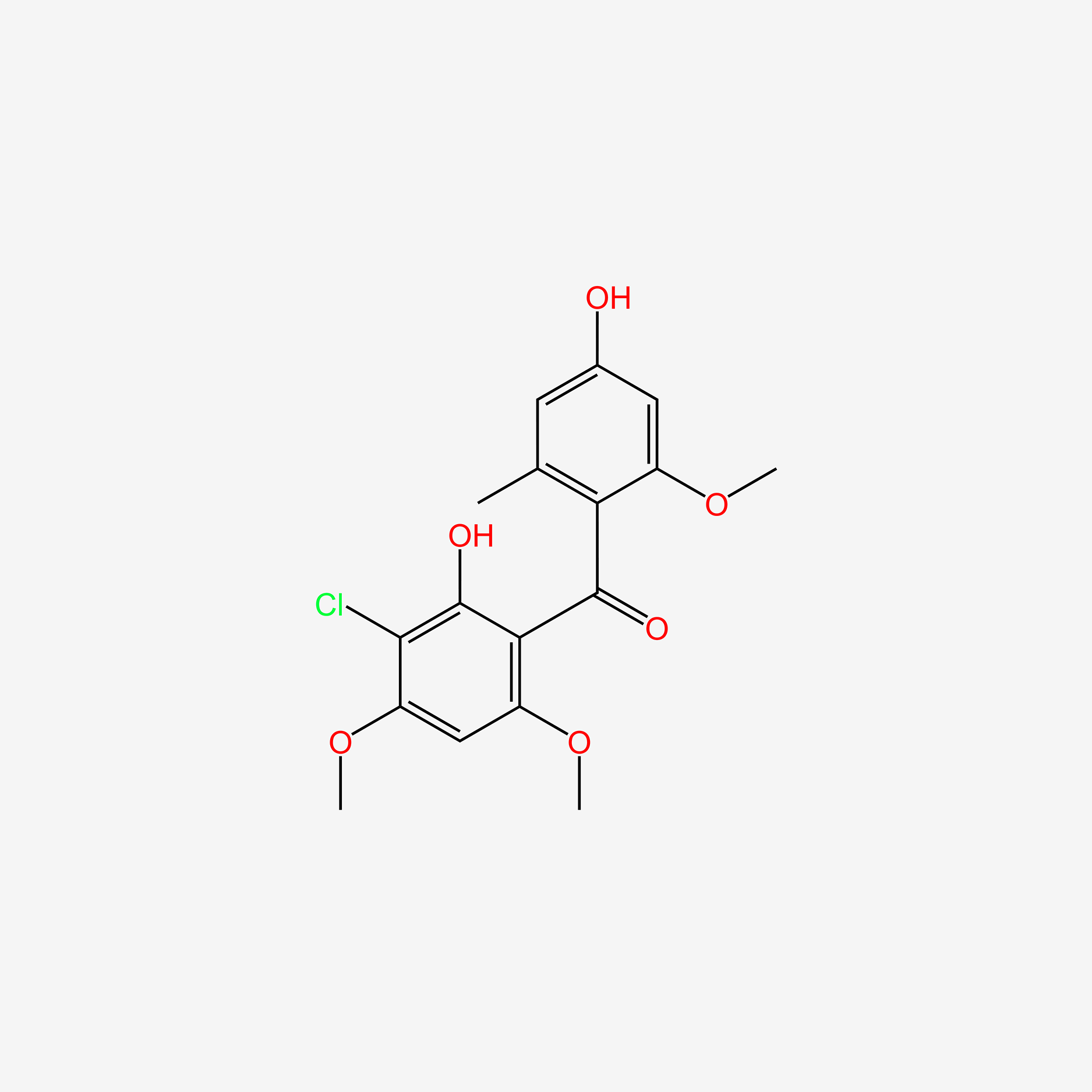 |
0.364 | D02LZB | 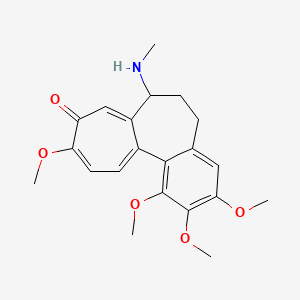 |
0.275 | ||
| ENC000478 | 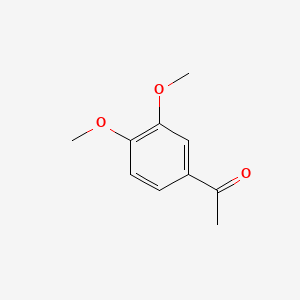 |
0.357 | D0Y7TS | 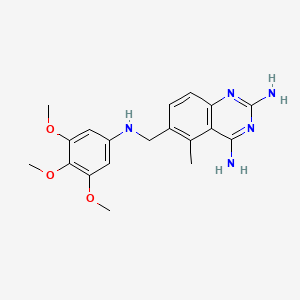 |
0.275 | ||
| ENC001379 | 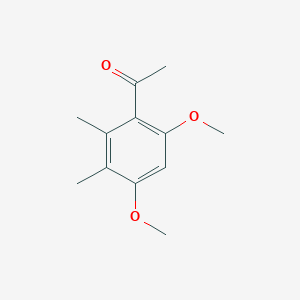 |
0.356 | D0E6OC | 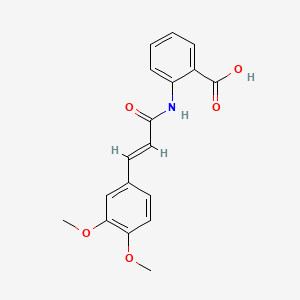 |
0.271 | ||