NPs Basic Information
|
Name |
Syringaldehyde
|
| Molecular Formula | C9H10O4 | |
| IUPAC Name* |
4-hydroxy-3,5-dimethoxybenzaldehyde
|
|
| SMILES |
COC1=CC(=CC(=C1O)OC)C=O
|
|
| InChI |
InChI=1S/C9H10O4/c1-12-7-3-6(5-10)4-8(13-2)9(7)11/h3-5,11H,1-2H3
|
|
| InChIKey |
KCDXJAYRVLXPFO-UHFFFAOYSA-N
|
|
| Synonyms |
SYRINGALDEHYDE; 134-96-3; 4-Hydroxy-3,5-dimethoxybenzaldehyde; 3,5-Dimethoxy-4-hydroxybenzaldehyde; Syringic aldehyde; Syringylaldehyde; Benzaldehyde, 4-hydroxy-3,5-dimethoxy-; Syringealdehyde; Gallaldehyde 3,5-dimethyl ether; Springaldehyde; 3,5-Dimethoxy-4-hydroxybenzene carbonal; 4-hydroxy-3,5-dimethoxy-benzaldehyde; Cedar aldehyde; Benzaldehyde, 3,5-dimethoxy-4-hydroxy-; MFCD00006943; NSC 41153; AI3-28796; 4-Hydroksy-3,5-dwumetoksybenzaldehyd [Polish]; 4-Hydroksy-3,5-dwumetoksybenzaldehyd; 2ZR01KTT21; CHEBI:67380; NSC-41153; EINECS 205-167-5; BRN 0784514; syringaldehye; UNII-2ZR01KTT21; Syringe aldehyde; Syringaldehyde, 98%; bmse000595; bmse010204; SYRINGALDEHYDE [MI]; SCHEMBL150376; Syringaldehyde, >=98%, FG; CHEMBL225303; DTXSID2059643; FEMA NO. 4049; 86220_FLUKA; Syringaldehyde, analytical standard; ZINC152926; HY-N1390; NSC41153; STR09162; AC7930; BBL023037; Benzaldehyde,5-dimethoxy-4-hydroxy-; s4765; STK801968; ZINC00152926; 4-hydroxy-3,5-dimethoxy benzaldehyde; 2,6-DIMETHOXY-4-FORMYLPHENOL; 3,5-Dimethoxy-4-hydroxy benzaldehyde; 4-FORMYL-2,6-DIMETHOXYPHENOL; AKOS000119539; AC-7993; CCG-266448; BP-12551; SY014703; CS-0016810; D0635; FT-0618631; EN300-21558; Syringaldehyde, Vetec(TM) reagent grade, 98%; 134S963; A887790; AP-065/41884112; Q411695; 4-HYDROXY-3,5-DIMETHOXYBENZALDEHYDE [FHFI]; syringaldehyde; 3,5-dimethoxy-4-hydroxybenzaldehyde; W-108274; F2190-0619; Z104501688
|
|
| CAS | 134-96-3 | |
| PubChem CID | 8655 | |
| ChEMBL ID | CHEMBL225303 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 182.17 | ALogp: | 0.0 |
| HBD: | 1 | HBA: | 4 |
| Rotatable Bonds: | 3 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 55.8 | Aromatic Rings: | 1 |
| Heavy Atoms: | 13 | QED Weighted: | 0.722 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.664 | MDCK Permeability: | 0.00001390 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.023 |
| Human Intestinal Absorption (HIA): | 0.033 | 20% Bioavailability (F20%): | 0.003 |
| 30% Bioavailability (F30%): | 0.011 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.865 | Plasma Protein Binding (PPB): | 81.10% |
| Volume Distribution (VD): | 0.646 | Fu: | 16.18% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.754 | CYP1A2-substrate: | 0.911 |
| CYP2C19-inhibitor: | 0.037 | CYP2C19-substrate: | 0.631 |
| CYP2C9-inhibitor: | 0.025 | CYP2C9-substrate: | 0.64 |
| CYP2D6-inhibitor: | 0.009 | CYP2D6-substrate: | 0.486 |
| CYP3A4-inhibitor: | 0.02 | CYP3A4-substrate: | 0.251 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 8.824 | Half-life (T1/2): | 0.904 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.029 | Human Hepatotoxicity (H-HT): | 0.023 |
| Drug-inuced Liver Injury (DILI): | 0.024 | AMES Toxicity: | 0.03 |
| Rat Oral Acute Toxicity: | 0.033 | Maximum Recommended Daily Dose: | 0.078 |
| Skin Sensitization: | 0.526 | Carcinogencity: | 0.029 |
| Eye Corrosion: | 0.977 | Eye Irritation: | 0.986 |
| Respiratory Toxicity: | 0.694 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
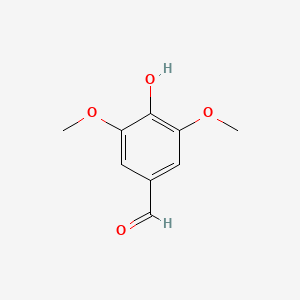
| ENC000367 | 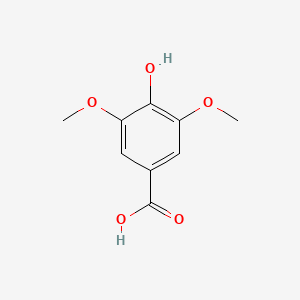 |
0.600 | D0E9CD |  |
0.444 | ||
| ENC004830 | 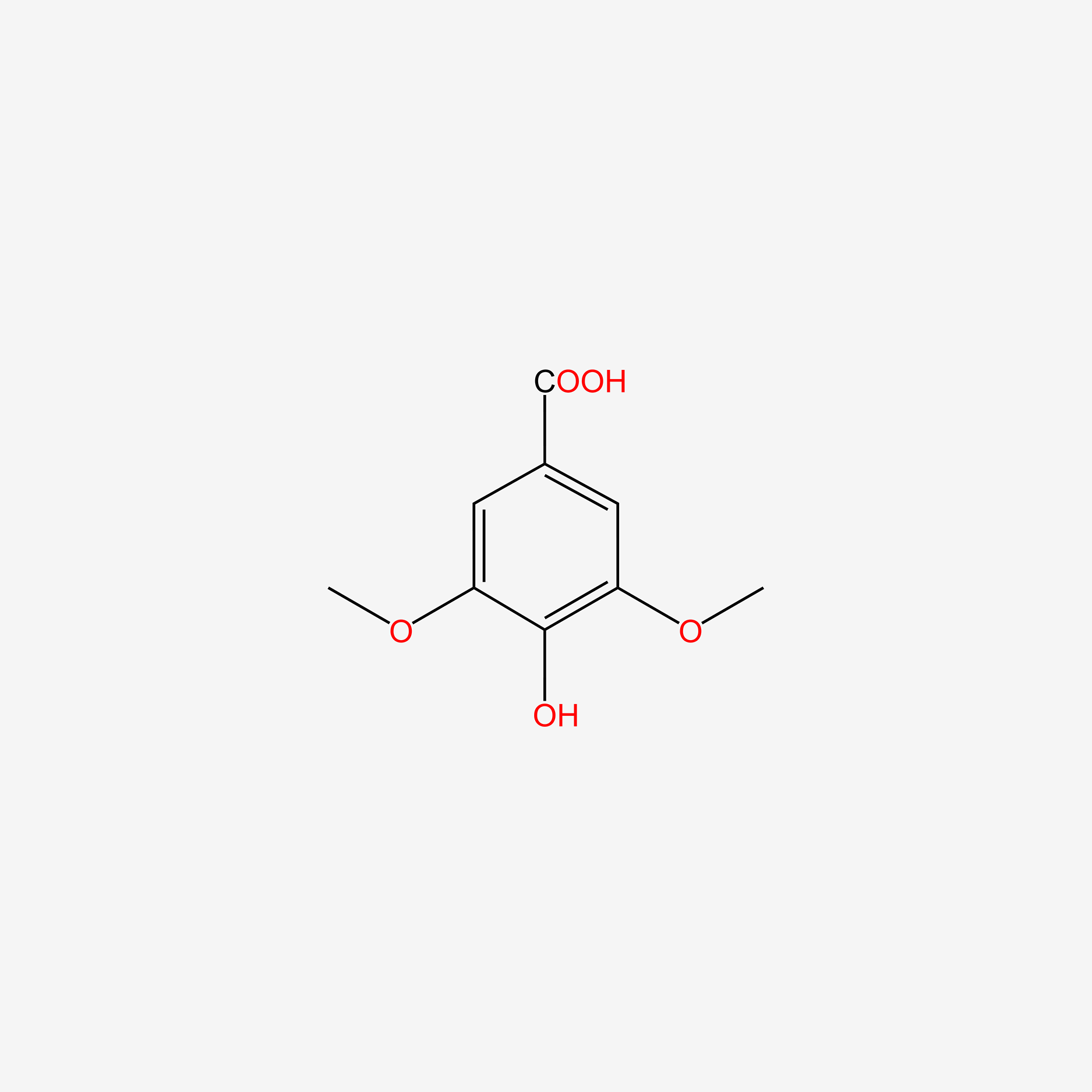 |
0.600 | D0E6OC |  |
0.308 | ||
| ENC000168 | 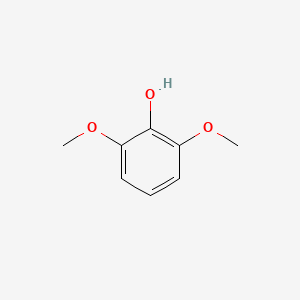 |
0.477 | D06GCK |  |
0.300 | ||
| ENC000068 | 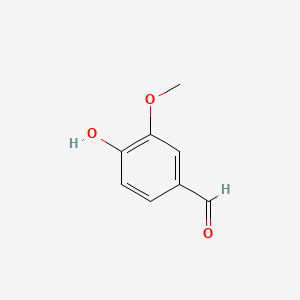 |
0.477 | D09GYT | 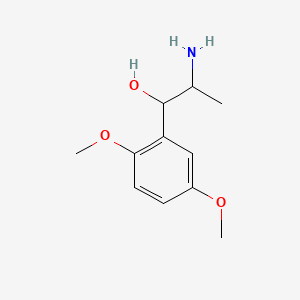 |
0.293 | ||
| ENC002285 | 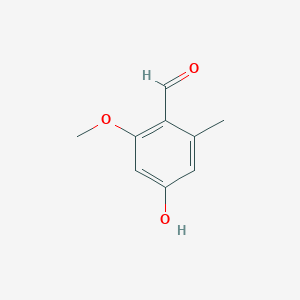 |
0.457 | D06QKV |  |
0.286 | ||
| ENC000501 | 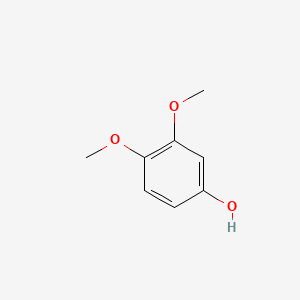 |
0.444 | D0AO5H | 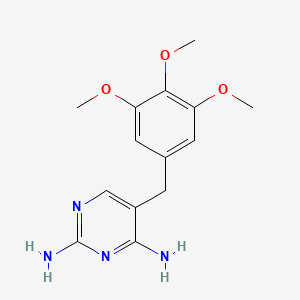 |
0.274 | ||
| ENC001461 | 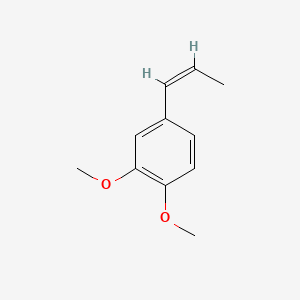 |
0.420 | D02XJY | 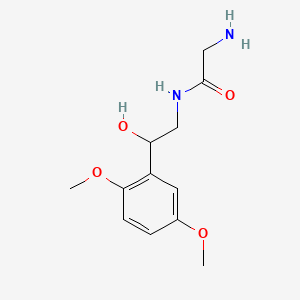 |
0.273 | ||
| ENC000671 | 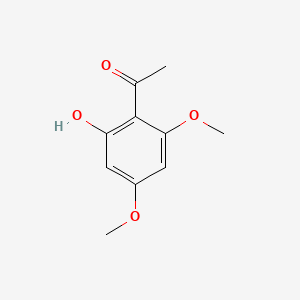 |
0.412 | D09PJX | 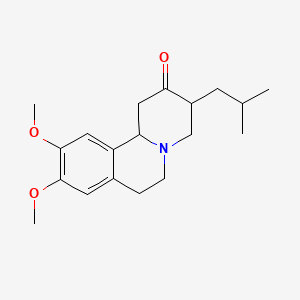 |
0.269 | ||
| ENC005411 | 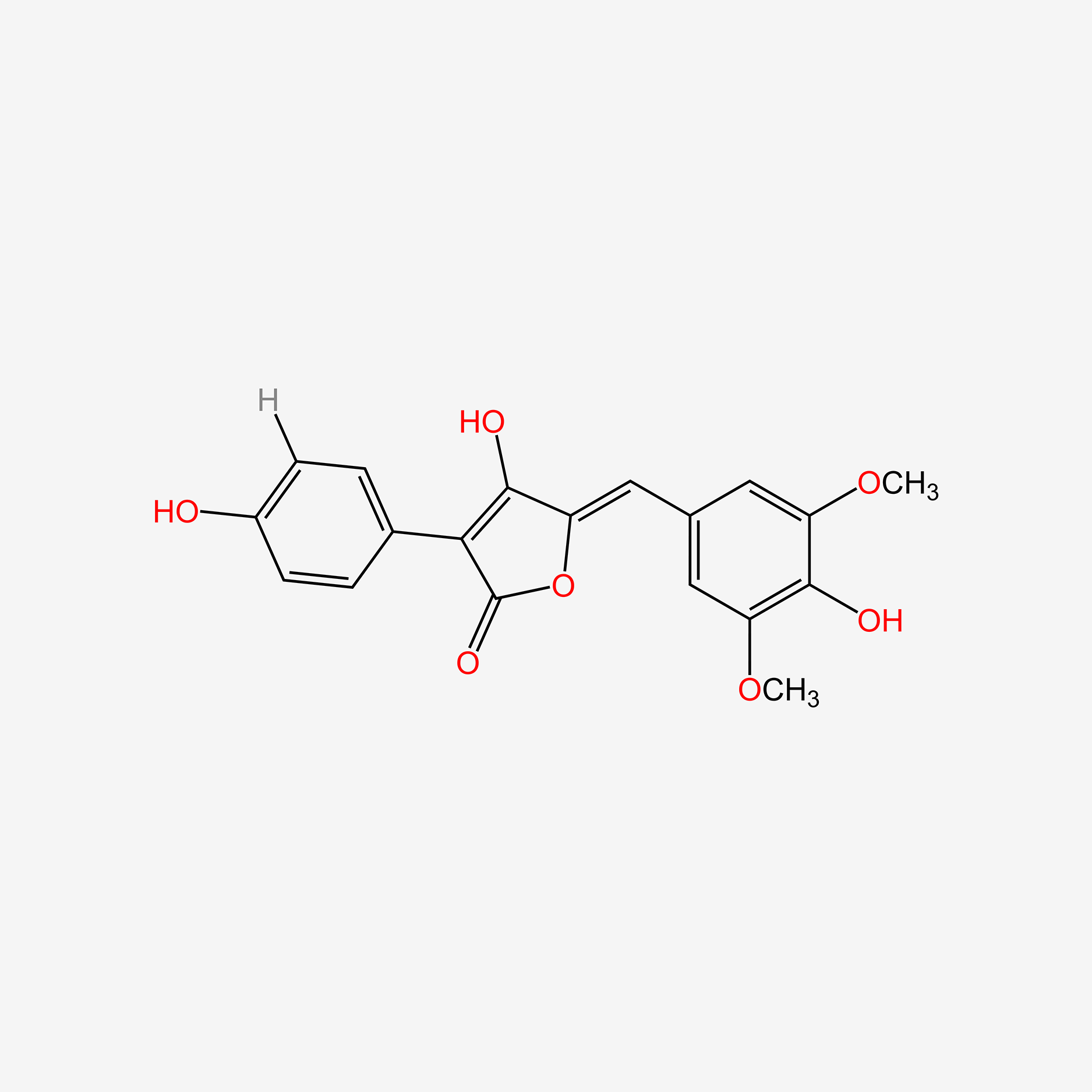 |
0.408 | D0Q9ON | 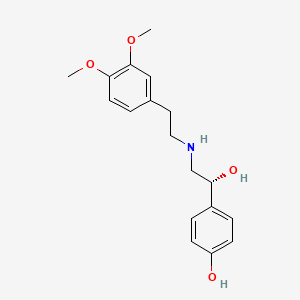 |
0.266 | ||
| ENC000764 |  |
0.400 | D0F4ZY |  |
0.258 | ||