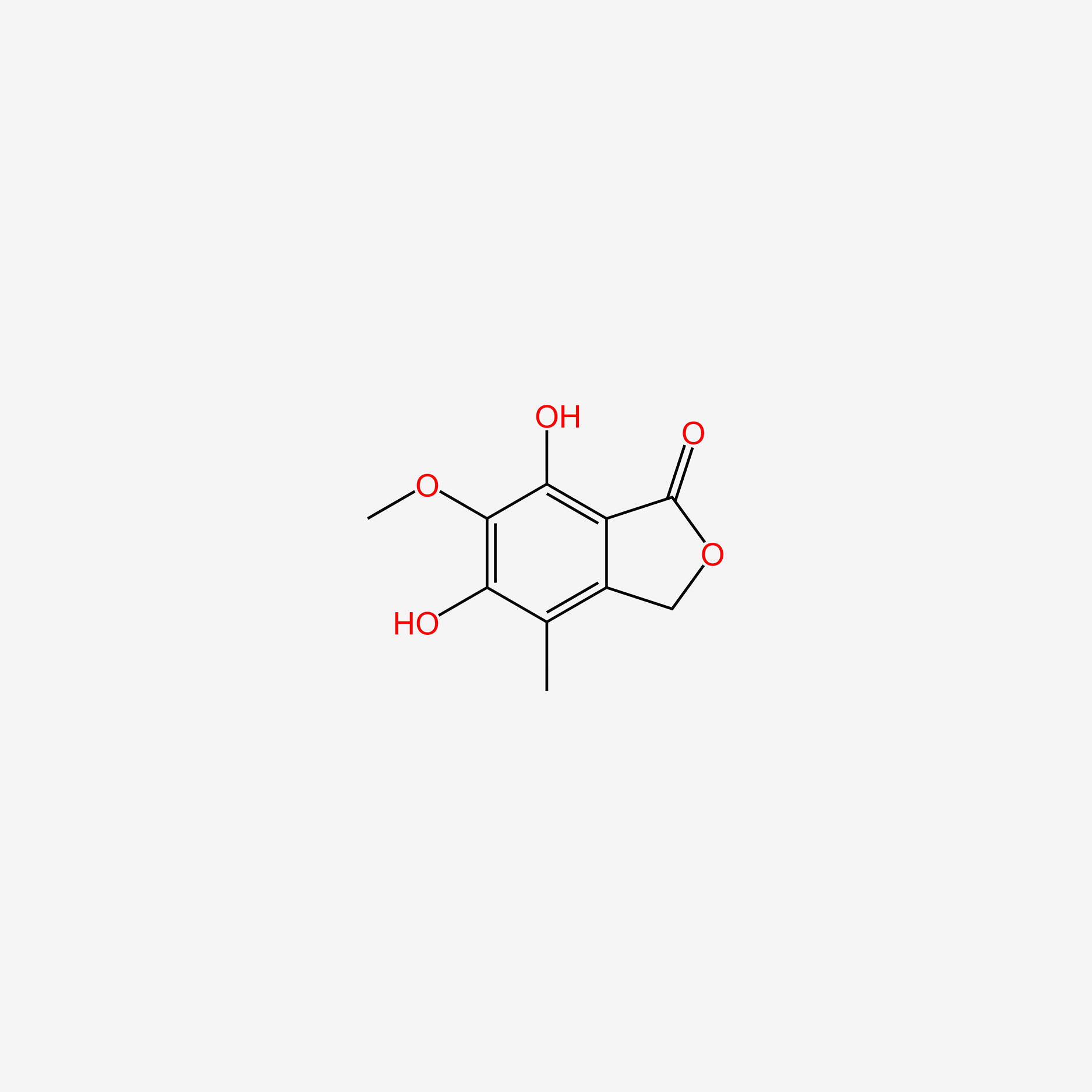
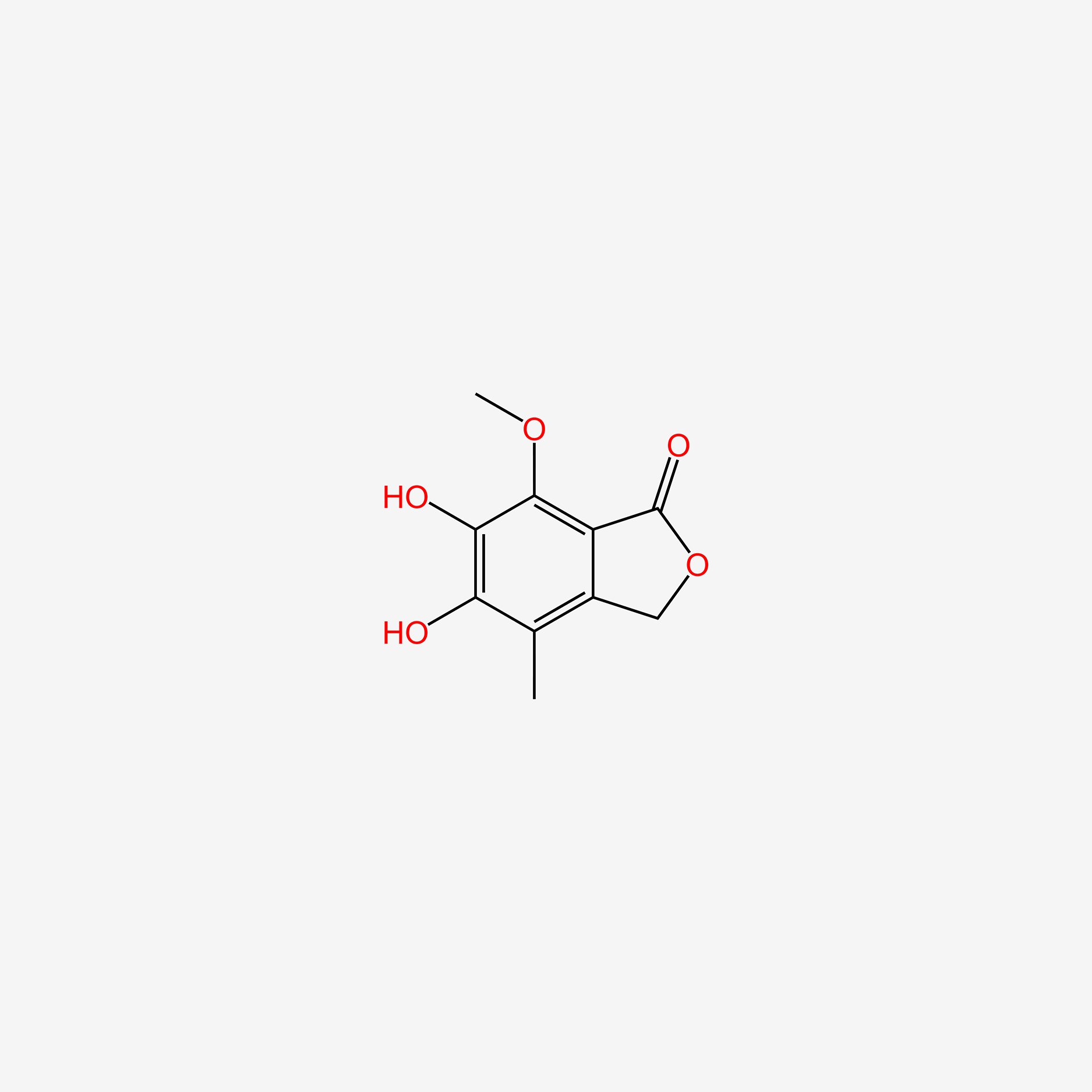
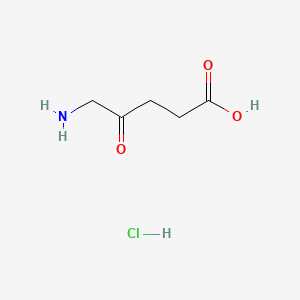
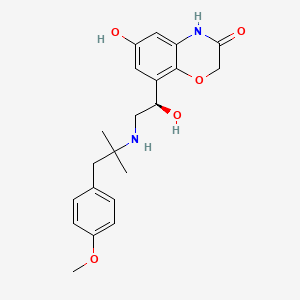
| InChI |
InChI=1S/C17H20O6/c1-9(5-7-13(18)19)4-6-11-15(20)14-12(8-23-17(14)21)10(2)16(11)22-3/h4,20H,5-8H2,1-3H3,(H,18,19)/b9-4+
|
| Synonyms |
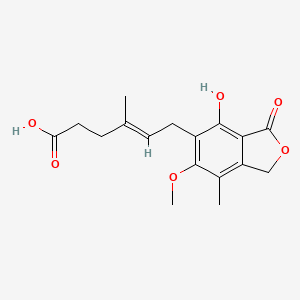
mycophenolic acid; 24280-93-1; Mycophenolate; Myfortic; Melbex; Mycophenolsaeure; 483-60-3; Lilly-68618; 6-(4-Hydroxy-6-methoxy-7-methyl-3-oxo-5-phthalanyl)-4-methyl-4-hexenoic acid; NSC-129185; Acido micofenolico; Micofenolico acido; (4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dihydro-2-benzofuran-5-yl)-4-methylhex-4-enoic acid; Acide mycophenolique; Acidum mycophenolicum; CCRIS 5565; NSC 129185; Ly 68618; (E)-6-(4-Hydroxy-6-methoxy-7-methyl-3-oxo-5-phthalanyl)-4-methyl-4-hexenoic acid; (E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dihydroisobenzofuran-5-yl)-4-methylhex-4-enoic acid; (E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1H-2-benzofuran-5-yl)-4-methylhex-4-enoic acid; MFCD00036814; Lilly 68618; 4-Hexenoic acid, 6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-, (E)-; CHEMBL866; HU9DX48N0T; 6-(1,3-Dihydro-7-hydroxy-5-methoxy-4-methyl-1-oxoisobenzofuran-6-yl)-4-methyl-4-hexanoic acid; CHEBI:168396; TNP00198; RS-61443 [AS MOFETIL]; NSC129185; 4-Hexenoic acid, 6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-5-phthalanyl)-4-methyl-, (E)-; 4-Methyl-5-methoxy-7-hydroxy-6-(5-carboxy-3-methylpent-2-en-1-yl)-phthalide (E)-; 6-(1,3-Dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methylhex-4-enoic acid; 6-(4-Hydroxy-6-methoxy-7-methyl-3-oxo-5-phthalanyl)-4-methyl-4-hexenoic acid (E)-; 4-Hexenoic acid, 6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-, (4E)-; NCGC00016786-01; CAS-24280-93-1; Mycophenolic acid 100 microg/mL in Acetonitrile; 6-(1,3-Dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-4-hexenoic acid; DSSTox_CID_21070; DSSTox_RID_79619; DSSTox_GSID_41070; Mycophenoic acid; 6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1H-isobenzofuran-5-yl)-4-methyl-4-hexenoic acid; Micofenolico acido [Spanish]; (4E)-6-(4-Hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dihydroisobenzofuran-5-yl)-4-methylhex-4-enoic Acid (Mycophenolic Acid); (E)-6-(1,3-Dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-4-hexenoic acid; 4-Hexenoic acid,6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-, (4E)-; 6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dihydroisobenzofuran-5-yl)-4-methylhex-4-enoic acid; MOA; SMR000471887; Acido micofenolico [INN-Spanish]; 6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dihydro-2-benzofuran-5-yl)-4-methylhex-4-enoic acid; Acide mycophenolique [INN-French]; Acidum mycophenolicum [INN-Latin]; SR-01000597602; EINECS 246-119-3; UNII-HU9DX48N0T; mycophenolic-acid; Prestwick_817; EINECS 207-595-8; Mycophenolic acid [USAN:INN:BAN]; Tocris-1505; (Z)-Mycophenolic Acid; 1jr1; starbld0040186; Mycophenolic acid (TN); Prestwick2_000556; Prestwick3_000556; Spectrum5_001654; Mycophenolic Acid (MPA); UPCMLD-DP028; 4-Hexenoic acid, (E)-; EC 246-119-3; Mycophenolate;RS-61443; SCHEMBL4549; Mycophenolic (Mycophenolate); Mycophenolic acid, >=98%; BSPBio_000631; BSPBio_002534; MYCOPHENOLATE [VANDF]; MLS001074701; MLS002222265; MLS002695945; BIDD:GT0456; SPECTRUM1500674; MYCOPHENOLIC ACID [MI]; BPBio1_000695; GTPL6832; MEGxm0_000120; Mycophenolic acid (USAN/INN); Myfortic (mycophenolate sodium); SCHEMBL2514376; ZINC1758; MYCOPHENOLIC ACID [INN]; DTXSID4041070; MYCOPHENOLIC ACID [USAN]; UPCMLD-DP028:001; ACon0_000960; ACon1_000496; BDBM19264; CHEBI:92545; HMS500H08; EX-A975; MYCOPHENOLIC ACID [VANDF]; MYCOPHENOLIC ACID [MART.]; HMS1569P13; HMS1921A18; HMS2089A17; HMS2092G22; HMS2096P13; HMS2268H22; HMS3403P09; HMS3412F09; HMS3676F09; Pharmakon1600-01500674; MYCOPHENOLIC ACID [WHO-DD]; ACT02623; AMY40494; BCP05321; HY-B0421; Tox21_110610; BBL034696; CCG-39914; HB3987; NSC757424; s2487; STL419986; Mycophenolic acid, analytical standard; AKOS015888214; Tox21_110610_1; AC-4491; BCP9000970; DB01024; DS-1638; MYCOPHENOLIC ACID [ORANGE BOOK]; NSC-757424; SDCCGMLS-0066618.P001; (E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1H-isobenzofuran-5-yl)-4-methyl-hex-4-enoic acid; IDI1_000146; NCGC00016786-02; NCGC00016786-03; NCGC00016786-04; NCGC00016786-05; NCGC00016786-06; NCGC00016786-07; NCGC00016786-08; NCGC00016786-09; NCGC00016786-10; NCGC00016786-11; NCGC00016786-12; NCGC00016786-15; NCGC00025190-01; NCGC00025190-02; NCGC00025190-03; NCGC00025190-04; NCGC00025190-05; NCGC00025190-07; NCGC00025190-08; NCGC00025190-09; NCGC00025190-10; (4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxohydroisobenzofuran-5-yl)-4-methylhex -4-enoic acid; BM164624; Mycophenolic Acid - CAS 24280-93-1; SBI-0051945.P003; A-249; M2216; SW196951-2; C20380; D05096; EN300-122327; M 5255; M02087; AB00052466-13; AB00052466-14; AB00052466_15; AB00052466_16; EN300-1273238; 280M931; A817192; Mycophenolate mofetil impurity, mycophenolic acid-; Q420553; SR-01000597602-1; SR-01000597602-3; SR-01000597602-4; BRD-K63750851-001-06-6; BRD-K63750851-001-13-2; MYCOPHENOLATE MOFETIL IMPURITY F [EP IMPURITY]; Z2315575694; MYCOPHENOLATE MOFETIL IMPURITY, MYCOPHENOLIC ACID- [USP IMPURITY]; Mycophenolic acid, powder, BioReagent, suitable for cell culture, >=98%; 6-(4-Hydroxy-6-methoxy-7-methyl-3-oxo-5-phthalanyl)-4-methyl-4-hexenoic acid;NSC 129185; 6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-5-phthanlanyl)-4-methyl-4-hexanoic acid; (E)-6-(1,3-dihydro-4-hydroxy-6- methoxy-7-methyl-3-oxoisobenzofuran-5-yl)-4-methyl-4-hexenoic acid; (E)-6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxoisobenzofuran-5-yl)-4-methyl-4-hexenoic acid; 1162256-90-7; 4-Hexenoic acid, 6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-, (4E)- (9CI); 4-Hexenoic acid, 6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-5-phthalanyl)-4-methyl-, (E)- (8CI); 4-Hexenoic acid,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5- isobenzofuranyl)-4-methyl-, (E)-; 4-Methyl-6-[3-oxo-7-methyl-4-hydroxy-6-methoxy-1,3-dihydroisobenzofuran-5-yl]-4-hexenoic acid; E-6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-4-hexenoic acid
|