NPs Basic Information
|
Name |
Docosahexaenoic Acid
|
| Molecular Formula | C22H32O2 | |
| IUPAC Name* |
(4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hexaenoic acid
|
|
| SMILES |
CC/C=C\C/C=C\C/C=C\C/C=C\C/C=C\C/C=C\CCC(=O)O
|
|
| InChI |
InChI=1S/C22H32O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22(23)24/h3-4,6-7,9-10,12-13,15-16,18-19H,2,5,8,11,14,17,20-21H2,1H3,(H,23,24)/b4-3-,7-6-,10-9-,13-12-,16-15-,19-18-
|
|
| InChIKey |
MBMBGCFOFBJSGT-KUBAVDMBSA-N
|
|
| Synonyms |
Docosahexaenoic acid; Doconexent; 6217-54-5; Cervonic acid; cis-4,7,10,13,16,19-Docosahexaenoic acid; Docosahexaenoate; (4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hexaenoic acid; all-cis-DHA; Doconexento; Doconexentum; Doxonexent; AquaGrow Advantage; all-Z-Docosahexaenoic acid; Doconexent [INN]; Martek DHA HM; Ropufa 60; all-cis-4,7,10,13,16,19-Docosahexaenoic acid; (4Z,7Z,10Z,13Z,16Z,19Z)-Docosahexaenoic acid; Docosaheaenoic-acid; Docosahexaenoic acid (all-Z); CCRIS 7670; all-cis-docosa-4,7,10,13,16,19-hexaenoic acid; ZAD9OKH9JC; DOCOSAHEXANOIC ACID; (all-Z)-4,7,10,13,16,19-Docosahexaenoic acid; DOCOSA-4,7,10,13,16,19-HEXAENOIC ACID; (4Z,7Z,10Z,13Z,16Z,19Z)-4,7,10,13,16,19-Docosahexaenoic acid; CHEMBL367149; docosahexaenoic acid(DHA); CHEBI:28125; delta4,7,10,13,16,19-Docosahexaenoic acid; 4-cis,7-cis,10-cis,13-cis,16-cis,19-cis-Docosahexaenoic acid; 4Z,7Z,10Z,13Z,16Z,19Z-docosahexaenoic acid; Docosahexaenoic acid (c22:6 n3); OMEGA-3 MARINE TRIGLYCERIDES; MFCD00065722; 4,7,10,13,16,19-Docosahexaenoic acid, (all-Z)-; FA 22:6; efalex; NCGC00161345-04; C22:6 (n-3); DOCOSAHEXAENOIC ACID (22:6 n-3); 22:6 n-3; 22:6(n-3); cis-4,7,10,13,16,19-Docosahexanoic acid; 4,7,10,13,16,19-Docosahexaenoic acid; C22:6n-3,6,9,12,15,18; Monolife 50; Marinol D 50TG; SR-05000002130; UNII-ZAD9OKH9JC; Doconexentum [INN-Latin]; Doconexento [INN-Spanish]; DHA-[21,21,22,22,22-d5]; Cervonate; DTXSID5040465; 1fdq; Algal DHA; Docohexanenoic Acid; Docosahexanenoic acid; all-Z-Docosahexaenoate; Spectrum5_002062; Docosahexaenoic acid (6CI); DSSTox_CID_20465; DSSTox_RID_79498; DSSTox_GSID_40465; SCHEMBL19577; BSPBio_001298; Docoshexaenoic Acid (Powder); MLS004773950; BML3-B02; GTPL1051; Retriacyl (proposed trade name); BCBcMAP01_000145; DOCOSAHEXAENOIC ACID [MI]; HMS1361A20; HMS1791A20; HMS1989A20; HMS3402A20; HMS3649J15; DOCOSAHEXAENOIC ACID [INCI]; HY-B2167; ZINC4474564; DOCOSAHEXAENOIC ACID [VANDF]; Tox21_111992; BDBM50210259; DOCOSAHEXAENOIC ACID [MART.]; LMFA01030185; DOCOSAHEXAENOIC ACID [USP-RS]; DOCOSAHEXAENOIC ACID [WHO-DD]; AKOS015962159; AC-1010; CCG-207958; CCG-208135; CS-6261; DB03756; KL-0761; IDI1_033768; 4,7,10,13,16,19-Docosahexaenoate; NCGC00161345-01; NCGC00161345-02; NCGC00161345-03; NCGC00161345-05; NCGC00161345-07; all cis- Docosahexaenoic acid (cis-DHA); SMR001881493; CAS-6217-54-5; cis-4,7,10,13,16,19-Docosahexanoate; D2226; S6454; C06429; H10987; AB01563379_01; DOCOSAHEXAENOIC ACID (DHA) (C22:6 N3); 217D545; Q423345; SR-05000002130-1; SR-05000002130-4; BRD-K39965020-001-02-6; 4,7,10,13,16,19-Docosahexaenoic acid, (all cis)-; A320050000; cis-4,7,10,13,16,19-Docosahexaenoic acid, >=98%; FA(22:6(4Z,7Z,10Z,13Z,16Z,19Z)); z,z,z,z,z,z-docosa-4,7,10,13,16,19-hexaenoic acid; (all-Z)-4,7,10,13,16,19-Docosahexaenoic Acid, DHA; 800E8E72-BBF4-46F7-A60B-B8F2B54669C7; C22H32O2 (cis-4,7,10,13,16,19-docosahexaenoic acid); 4,7,10,13,16,19-Docosahexaenoic acid, (all-Z)- (8CI); cis-4,7,10,13,16,19-Docosahexaenoic acid, analytical standard; Docosa-4Z,7Z,10Z,13Z,16Z,19Z-hexaenoic Acid (22:6, n-3); (4Z,7Z,10Z,13Z,16Z, 19Z)-docosa-4,7,10,13,16,19-hexaenoic acid; (4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4, 7,10,13,16,19-hexaenoic acid; (4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19- hexaenoic acid; cis-4,7,13,16,19-Docosahexaenoic acid (stabilized with vitamine E); 4,7,10,13,16,19-Docosahexaenoic acid, (4Z,7Z,10Z,13Z,16Z,19Z)- (9CI); 1024594-51-1; 25377-50-8; cis-4,7,10,13,16,19-Docosahexaenoic acid, 500 mug/mL in ethanol, certified reference material
|
|
| CAS | 6217-54-5 | |
| PubChem CID | 445580 | |
| ChEMBL ID | CHEMBL367149 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 328.5 | ALogp: | 6.2 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 14 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 37.3 | Aromatic Rings: | 0 |
| Heavy Atoms: | 24 | QED Weighted: | 0.369 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.304 | MDCK Permeability: | 0.00039038 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.058 | 20% Bioavailability (F20%): | 1 |
| 30% Bioavailability (F30%): | 1 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0 | Plasma Protein Binding (PPB): | 100.76% |
| Volume Distribution (VD): | 0.851 | Fu: | 0.82% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.069 | CYP1A2-substrate: | 0.871 |
| CYP2C19-inhibitor: | 0.082 | CYP2C19-substrate: | 0.121 |
| CYP2C9-inhibitor: | 0.112 | CYP2C9-substrate: | 0.991 |
| CYP2D6-inhibitor: | 0.18 | CYP2D6-substrate: | 0.962 |
| CYP3A4-inhibitor: | 0.131 | CYP3A4-substrate: | 0.081 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 1.999 | Half-life (T1/2): | 0.949 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.031 | Human Hepatotoxicity (H-HT): | 0.286 |
| Drug-inuced Liver Injury (DILI): | 0.002 | AMES Toxicity: | 0.979 |
| Rat Oral Acute Toxicity: | 0 | Maximum Recommended Daily Dose: | 0.532 |
| Skin Sensitization: | 0.964 | Carcinogencity: | 0.918 |
| Eye Corrosion: | 0.024 | Eye Irritation: | 0.301 |
| Respiratory Toxicity: | 0.876 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
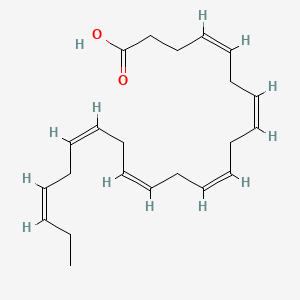
| ENC001103 | 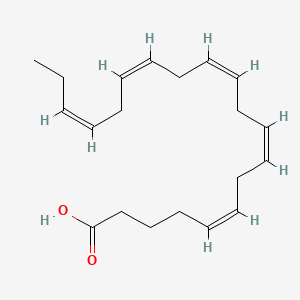 |
0.808 | D0Q5XX | 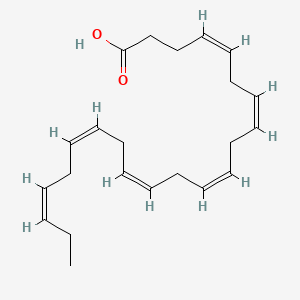 |
1.000 | ||
| ENC001094 | 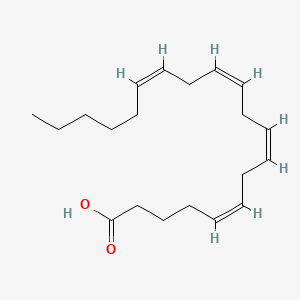 |
0.571 | D0G2MW | 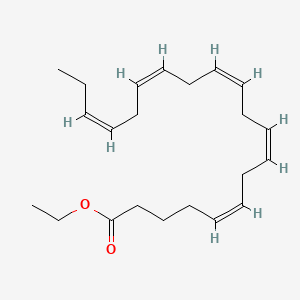 |
0.663 | ||
| ENC001549 | 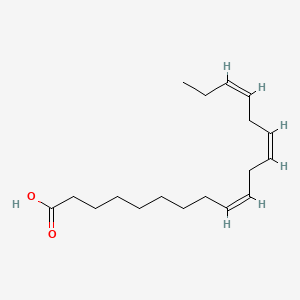 |
0.518 | D0UE9X | 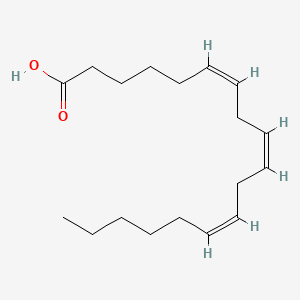 |
0.465 | ||
| ENC001945 | 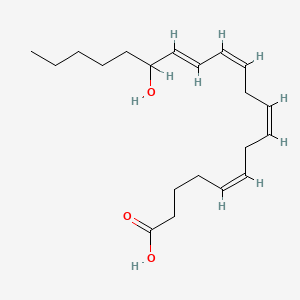 |
0.426 | D0O1TC | 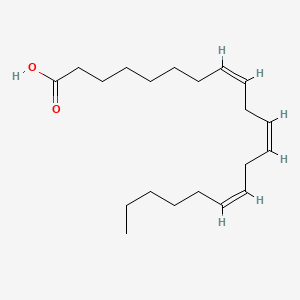 |
0.435 | ||
| ENC001661 | 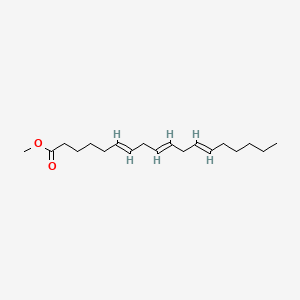 |
0.387 | D0PS6X | 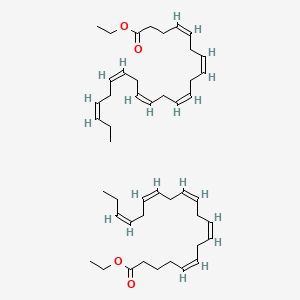 |
0.430 | ||
| ENC001857 | 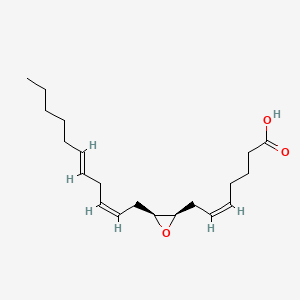 |
0.364 | D0G7WY |  |
0.365 | ||
| ENC001584 |  |
0.340 | D06FEA |  |
0.232 | ||
| ENC001535 |  |
0.340 | D0OR6A |  |
0.230 | ||
| ENC001662 |  |
0.322 | D03ZFG |  |
0.228 | ||
| ENC001920 |  |
0.286 | D0O1PH |  |
0.216 | ||