NPs Basic Information
|
Name |
Arachidonic Acid
|
| Molecular Formula | C20H32O2 | |
| IUPAC Name* |
(5Z,8Z,11Z,14Z)-icosa-5,8,11,14-tetraenoic acid
|
|
| SMILES |
CCCCC/C=C\C/C=C\C/C=C\C/C=C\CCCC(=O)O
|
|
| InChI |
InChI=1S/C20H32O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20(21)22/h6-7,9-10,12-13,15-16H,2-5,8,11,14,17-19H2,1H3,(H,21,22)/b7-6-,10-9-,13-12-,16-15-
|
|
| InChIKey |
YZXBAPSDXZZRGB-DOFZRALJSA-N
|
|
| Synonyms |
arachidonic acid; 506-32-1; arachidonate; (5Z,8Z,11Z,14Z)-icosa-5,8,11,14-tetraenoic acid; Immunocytophyte; (all-Z)-5,8,11,14-Eicosatetraenoic acid; cis-5,8,11,14-Eicosatetraenoic acid; 5,8,11,14-Eicosatetraenoic acid, (all-Z)-; 5Z,8Z,11Z,14Z-eicosatetraenoic acid; all-cis-5,8,11,14-eicosatetraenoic acid; (5Z,8Z,11Z,14Z)-5,8,11,14-Eicosatetraenoic Acid; CHEMBL15594; CHEBI:15843; 27YG812J1I; Icosa-5,8,11,14-tetraenoic acid; 5Z,8Z,11Z,14Z-icosatetraenoic acid; FA 20:4; ARACHIDONIC ACID (20:4, n-6); 5,8,11,14-Eicosatetraenoic acid; C20:4; [1-14C]Arachidonic acid; (14C)Arachidonic acid; Arachidonic Acid, 99%; Arachidonicacid; Arachidonsaeure; Immunocytophyt; Vevodar; UNII-27YG812J1I; arachidonic-acid; CCRIS 6312; 1adl; 1gnj; 1vyg; EINECS 208-033-4; MFCD00004417; AI3-09613; (14C)-arachidonic acid; Spectrum5_001910; Arachidonic Acid, >97%; SCHEMBL16162; BSPBio_001539; MLS001361328; ARACHIDONIC ACID [MI]; (5Z,8Z,11Z,14Z)-5,8,11,14-Eikosatetraensaeure; BML3-B03; GTPL2391; ARACHIDONIC ACID [INCI]; 5,8,11,14-Eicosatetraenoate; DTXSID4040420; BDBM22319; ARACHIDONIC ACID [MART.]; ARACHIDONIC ACID [WHO-DD]; CHEBI:137828; HMS1361M21; HMS1791M21; HMS1989M21; HMS3402M21; HMS3649B05; ZINC4474696; 5Z,8Z,11Z,14Z-Eicosatetraenoate; Arachidonic acid, >95.0% (GC); 5,8,11,14-Eicosatetraenoic acid, labeled with carbon-14, (all-Z)-; Arachidonic acid, analytical standard; cis-D5,8,11,14-Eicosatetraenoate; LMFA01030001; s6185; AKOS015950830; CCG-214838; DB04557; FS-5880; 5,8,11,14-all-cis-Eicosatetraenoate; all-cis-5,8,11,14-Eicosatetraenoate; ARACHIDONIC ACID (20:4 n-6); IDI1_034009; cis-D5,8,11,14-Eicosatetraenoic acid; NCGC00094608-01; NCGC00094608-02; NCGC00094608-03; NCGC00094608-04; NCGC00094608-05; NCGC00094608-06; (5Z,8Z,11Z,14Z)-Icosatetraenoic acid; (all-Z)-5,8,11,14-Eicosatetraenoate; 93444-49-6; AC-14348; AC-33769; SMR000857374; 5,8,11,14-all-cis-Eicosatetraenoic acid; HY-109590; A0781; all-cis-eicosa-5,8,11,14-tetraenoic acid; CS-0032762; cis-Delta(5,8,11,14)-eicosatetraenoic acid; 5-cis,8-cis,11-cis,14-cis-Eicosatetraenoate; Arachidonic acid (in Tocrisolvetrade mark100); C00219; W15452; FA(20:4(5Z,8Z,11Z,14Z)); 5-cis,8-cis,11-cis,14-cis-Eicosatetraenoic acid; 506A321; A929392; Q407699; SR-01000838311; SR-01000838311-2; BRD-K03070961-001-02-8; BRD-K03070961-001-04-4; (5Z,8Z,11Z,14Z)-5,8,11,14-Icosatetraenoic acid; (5Z,8Z,11Z,14Z)-icosa-5,8,11,14-tetraenoicacid; (5Z,8Z,11Z,14Z)-5,8,11,14-Icosatetraenoic acid #; Arachidonic acid, from non-animal source, >=98.5% (GC); Arachidonic acid, from porcine liver, >=99% (GC), liquid; D18DBC10-379C-4E78-9A50-8B791A2F4E68; Arachidonic acid, from porcine liver, >=85% (capillary GC), liquid; Arachidonic acid, 1.0 mg/mL in ethanol, certified reference material; Arachidonic acid, from porcine liver, BioReagent, suitable for cell culture, >=99% (GC)
|
|
| CAS | 506-32-1 | |
| PubChem CID | 444899 | |
| ChEMBL ID | CHEMBL15594 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 304.5 | ALogp: | 6.3 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 14 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 37.3 | Aromatic Rings: | 0 |
| Heavy Atoms: | 22 | QED Weighted: | 0.305 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.263 | MDCK Permeability: | 0.00007840 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.033 | 20% Bioavailability (F20%): | 1 |
| 30% Bioavailability (F30%): | 1 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.002 | Plasma Protein Binding (PPB): | 99.85% |
| Volume Distribution (VD): | 0.735 | Fu: | 0.57% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.205 | CYP1A2-substrate: | 0.857 |
| CYP2C19-inhibitor: | 0.114 | CYP2C19-substrate: | 0.166 |
| CYP2C9-inhibitor: | 0.272 | CYP2C9-substrate: | 0.991 |
| CYP2D6-inhibitor: | 0.149 | CYP2D6-substrate: | 0.939 |
| CYP3A4-inhibitor: | 0.096 | CYP3A4-substrate: | 0.07 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 3.007 | Half-life (T1/2): | 0.916 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.026 | Human Hepatotoxicity (H-HT): | 0.251 |
| Drug-inuced Liver Injury (DILI): | 0.006 | AMES Toxicity: | 0.945 |
| Rat Oral Acute Toxicity: | 0.003 | Maximum Recommended Daily Dose: | 0.349 |
| Skin Sensitization: | 0.959 | Carcinogencity: | 0.783 |
| Eye Corrosion: | 0.147 | Eye Irritation: | 0.409 |
| Respiratory Toxicity: | 0.855 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
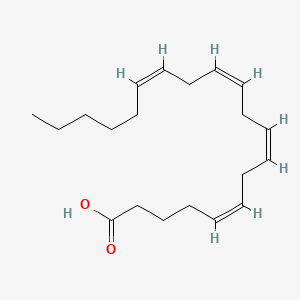
| ENC001103 | 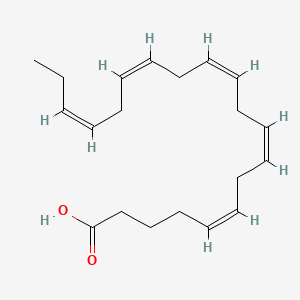 |
0.703 | D0UE9X | 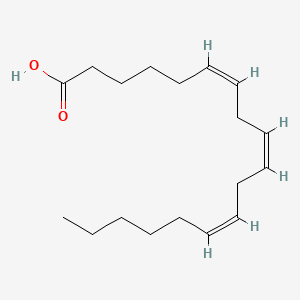 |
0.818 | ||
| ENC001549 | 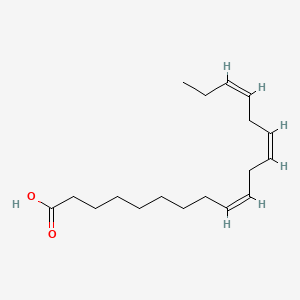 |
0.667 | D0O1TC | 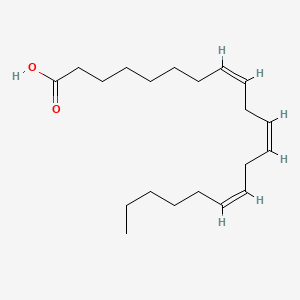 |
0.750 | ||
| ENC001661 | 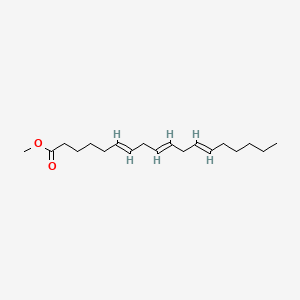 |
0.662 | D0G2MW | 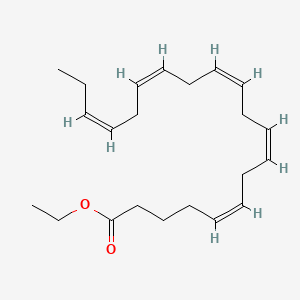 |
0.571 | ||
| ENC001857 | 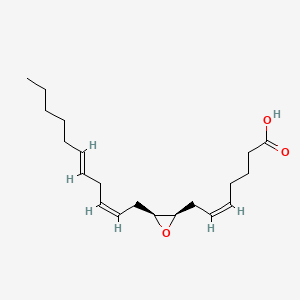 |
0.654 | D0Q5XX |  |
0.571 | ||
| ENC001945 |  |
0.641 | D0O1PH |  |
0.418 | ||
| ENC001584 |  |
0.622 | D0OR6A |  |
0.398 | ||
| ENC001535 | 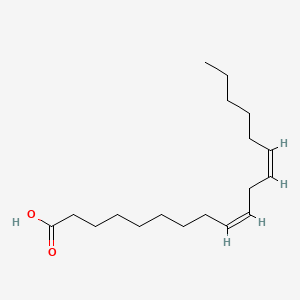 |
0.622 | D06FEA | 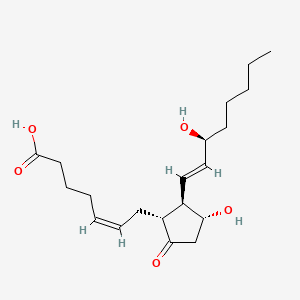 |
0.361 | ||
| ENC001098 | 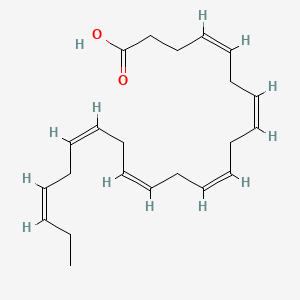 |
0.571 | D09SRR | 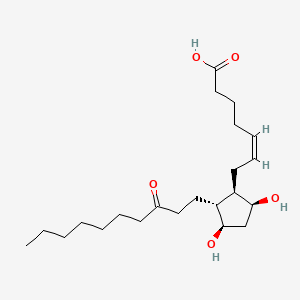 |
0.340 | ||
| ENC001554 | 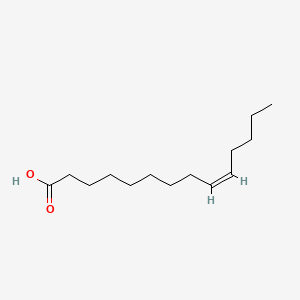 |
0.521 | D0PS6X | 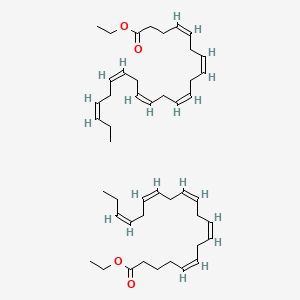 |
0.327 | ||
| ENC001920 | 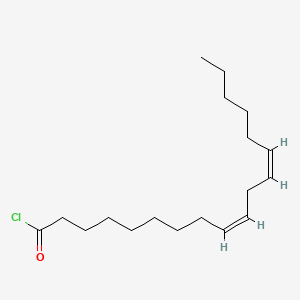 |
0.519 | D04RGA | 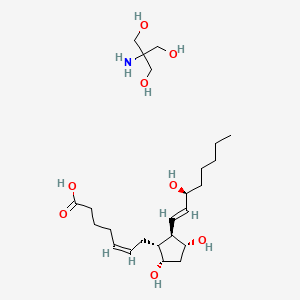 |
0.322 | ||