NPs Basic Information
|
Name |
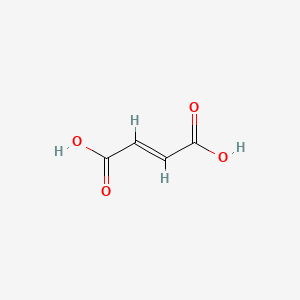
Fumaric Acid
|
| Molecular Formula | C4H4O4 | |
| IUPAC Name* |
(E)-but-2-enedioic acid
|
|
| SMILES |
C(=C/C(=O)O)\C(=O)O
|
|
| InChI |
InChI=1S/C4H4O4/c5-3(6)1-2-4(7)8/h1-2H,(H,5,6)(H,7,8)/b2-1+
|
|
| InChIKey |
VZCYOOQTPOCHFL-OWOJBTEDSA-N
|
|
| Synonyms |
fumaric acid; 110-17-8; trans-Butenedioic acid; fumarate; Allomaleic acid; Lichenic acid; (2E)-but-2-enedioic acid; Boletic acid; 2-Butenedioic acid; Tumaric acid; trans-1,2-Ethylenedicarboxylic acid; But-2-enedioic acid; Allomalenic acid; trans-2-Butenedioic acid; (E)-2-Butenedioic acid; 2-Butenedioic acid, (E)-; Fumaricum acidum; 2-Butenedioic acid (E)-; Kyselina fumarova; USAF EK-P-583; FEMA No. 2488; Butenedioic acid; Butenedioic acid, (E)-; (2E)-2-butenedioic acid; (E)-but-2-enedioic acid; 2-Butenedioic acid (2E)-; Caswell No. 465E; NSC-2752; FEMA Number 2488; 2-(E)-Butenedioic acid; 1,2-Ethylenedicarboxylic acid, (E); CCRIS 1039; HSDB 710; Allomaleic-acid; U-1149; Boletic-acid; trans-but-2-enedioic acid; 1,2-Ethenedicarboxylic acid, trans-; EPA Pesticide Chemical Code 051201; Fumaric acid (NF); Fumaric acid [NF]; (E)-Butenedioic acid; AI3-24236; 6915-18-0; INS NO.297; CHEBI:18012; INS-297; NSC2752; FC 33; 88XHZ13131; fum; E297; Lichenic acid (VAN); E-297; DSSTox_CID_1518; (E)-2-Butenedioate; DSSTox_RID_76195; DSSTox_GSID_21518; Fumaric acid 1000 microg/mL in Acetonitrile:Water; Kyselina fumarova [Czech]; (2E)-but-2-enedioate; Fumarsaeure; Donitic acid; but-2-enedioicacid; 9003-16-1; CAS-110-17-8; fumarate, 10; E-2-Butenedioic acid; Fumaric acid (8CI); EINECS 203-743-0; FC 33 (acid); Futrans-2-Butenedioic Acid; BRN 0605763; Allomaleate; Boletate; Lichenate; fumeric acid; UNII-88XHZ13131; trans-Butenedioate; NCGC00091192-02; Fumaric Acid,(S); Maleic Acid (MA); MFCD00002700; trans-2-Butenedioate; Fumaric Acid (FA); 2-(E)-Butenedioate; Fumaric acid, 99%; ethylenedicarboxylic acid; (Trans)-butenedioic acid; F0067; Fumaric acid, >=99%; bmse000083; FUMARIC ACID [II]; FUMARIC ACID [MI]; EC 203-743-0; WLN: QV1U1VQ-T; FUMARIC ACID [FCC]; SCHEMBL1177; FUMARIC ACID [FHFI]; FUMARIC ACID [HSDB]; FUMARIC ACID [INCI]; FUMARIC ACID [VANDF]; 4-02-00-02202 (Beilstein Handbook Reference); MLS002454406; 2-butenedioic acid, (2E)-; FUMARIC ACID [MART.]; (2E)-2-Butenedioic acid #; FUMARIC ACID [USP-RS]; FUMARIC ACID [WHO-DD]; CHEMBL503160; Fumaric Acid (Fragrance Grade); FUMARICUM ACIDUM [HPUS]; trans-1,2-Ethylenedicarboxylate; DTXSID3021518; BDBM26122; CHEBI:22958; 2(TRANS)-BUTENEDIOIC ACID; HMS2270C12; Pharmakon1600-01301022; Fumaric acid, >=99.0% (T); AMY30339; FUMARIC ACID [USP IMPURITY]; STR02646; ZINC3860193; Tox21_201769; Tox21_302826; 2-Butenedioic acid (2E)- (9CI); Fumaric acid, >=99%, FCC, FG; NSC760395; s4952; AKOS000118896; Fumaric acid, qNMR Standard for DMSO; CCG-266065; CS-W016599; DB01677; HY-W015883; NSC-760395; NCGC00091192-01; NCGC00091192-03; NCGC00256360-01; NCGC00259318-01; BP-13087; Fumaric acid, tested according to USP/NF; LS-12976; SMR000112117; Fumaric acid, puriss., >=99.5% (T); MALIC ACID IMPURITY A [EP IMPURITY]; EN300-17996; Fumaric acid, Vetec(TM) reagent grade, 99%; 1, (E); C00122; D02308; D85166; Q139857; Fumaric acid, BioReagent, suitable for cell culture; J-002389; Fumarate; 2-Butenedioic acid; Trans-Butenedioic acid; SODIUM AUROTHIOMALATE IMPURITY B [EP IMPURITY]; Z57127460; F8886-8257; Fumaric acid, certified reference material, TraceCERT(R); 26B3632D-E93F-4655-90B0-3C17855294BA; Fumaric acid, anhydrous, free-flowing, Redi-Dri(TM), >=99%; Fumaric acid, European Pharmacopoeia (EP) Reference Standard; Fumaric acid, United States Pharmacopeia (USP) Reference Standard; Fumaric Acid, Pharmaceutical Secondary Standard; Certified Reference Material; 623158-97-4
|
|
| CAS | 110-17-8 | |
| PubChem CID | 444972 | |
| ChEMBL ID | CHEMBL503160 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 116.07 | ALogp: | -0.3 |
| HBD: | 2 | HBA: | 4 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 74.6 | Aromatic Rings: | 0 |
| Heavy Atoms: | 8 | QED Weighted: | 0.503 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.65 | MDCK Permeability: | 0.00002210 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.044 |
| Human Intestinal Absorption (HIA): | 0.107 | 20% Bioavailability (F20%): | 0.016 |
| 30% Bioavailability (F30%): | 0.996 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.094 | Plasma Protein Binding (PPB): | 39.78% |
| Volume Distribution (VD): | 0.447 | Fu: | 31.86% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.01 | CYP1A2-substrate: | 0.045 |
| CYP2C19-inhibitor: | 0.031 | CYP2C19-substrate: | 0.034 |
| CYP2C9-inhibitor: | 0.102 | CYP2C9-substrate: | 0.314 |
| CYP2D6-inhibitor: | 0.052 | CYP2D6-substrate: | 0.123 |
| CYP3A4-inhibitor: | 0.017 | CYP3A4-substrate: | 0.004 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 1.982 | Half-life (T1/2): | 0.905 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.001 | Human Hepatotoxicity (H-HT): | 0.853 |
| Drug-inuced Liver Injury (DILI): | 0.743 | AMES Toxicity: | 0.027 |
| Rat Oral Acute Toxicity: | 0.255 | Maximum Recommended Daily Dose: | 0.004 |
| Skin Sensitization: | 0.879 | Carcinogencity: | 0.03 |
| Eye Corrosion: | 0.991 | Eye Irritation: | 0.45 |
| Respiratory Toxicity: | 0.354 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001541 | 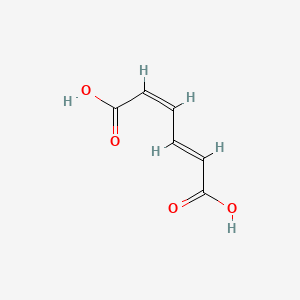 |
0.643 | D06VNK | 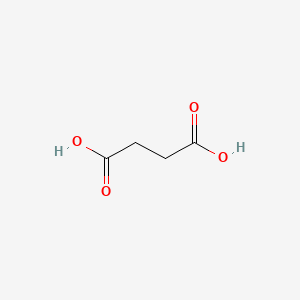 |
0.333 | ||
| ENC004134 | 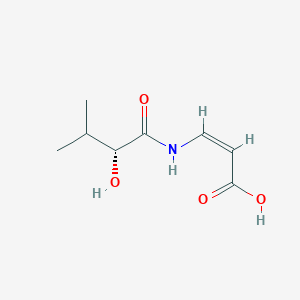 |
0.359 | D0G4JI | 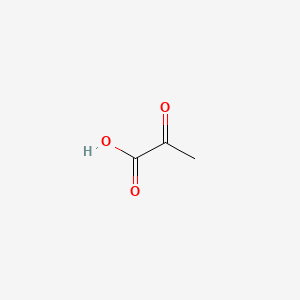 |
0.320 | ||
| ENC003852 | 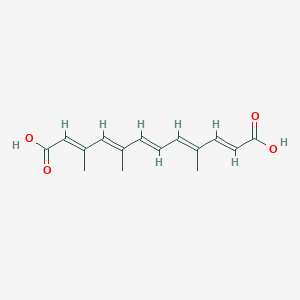 |
0.346 | D01GYK | 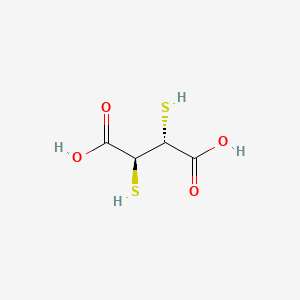 |
0.294 | ||
| ENC000062 | 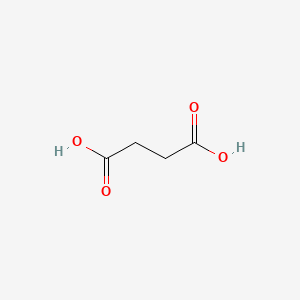 |
0.333 | D00ENY | 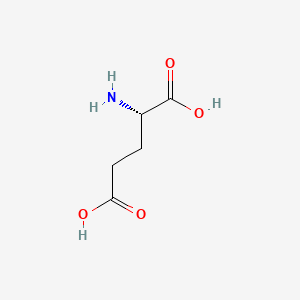 |
0.286 | ||
| ENC001586 | 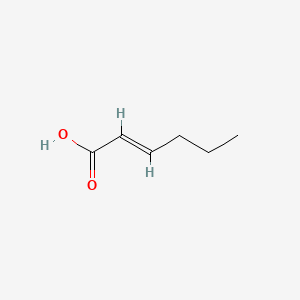 |
0.323 | D0A7MY | 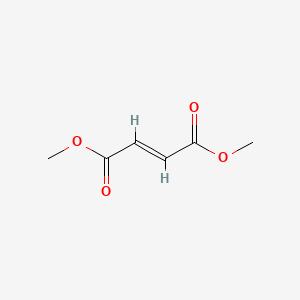 |
0.278 | ||
| ENC000061 | 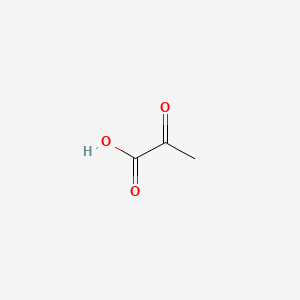 |
0.320 | D05ZGQ | 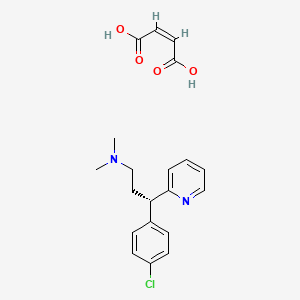 |
0.270 | ||
| ENC000044 | 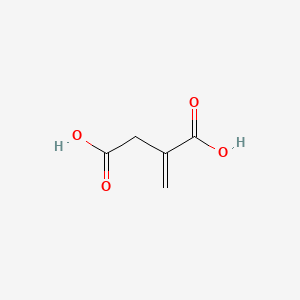 |
0.313 | D0RN2W | 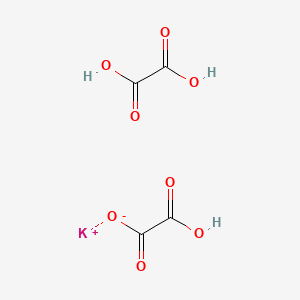 |
0.270 | ||
| ENC000148 | 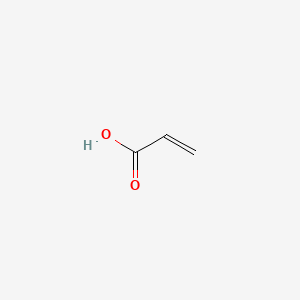 |
0.292 | D0X5SI | 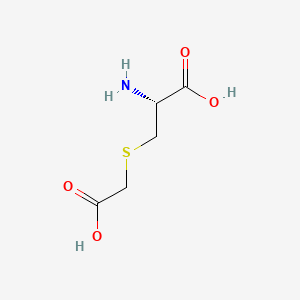 |
0.263 | ||
| ENC006057 | 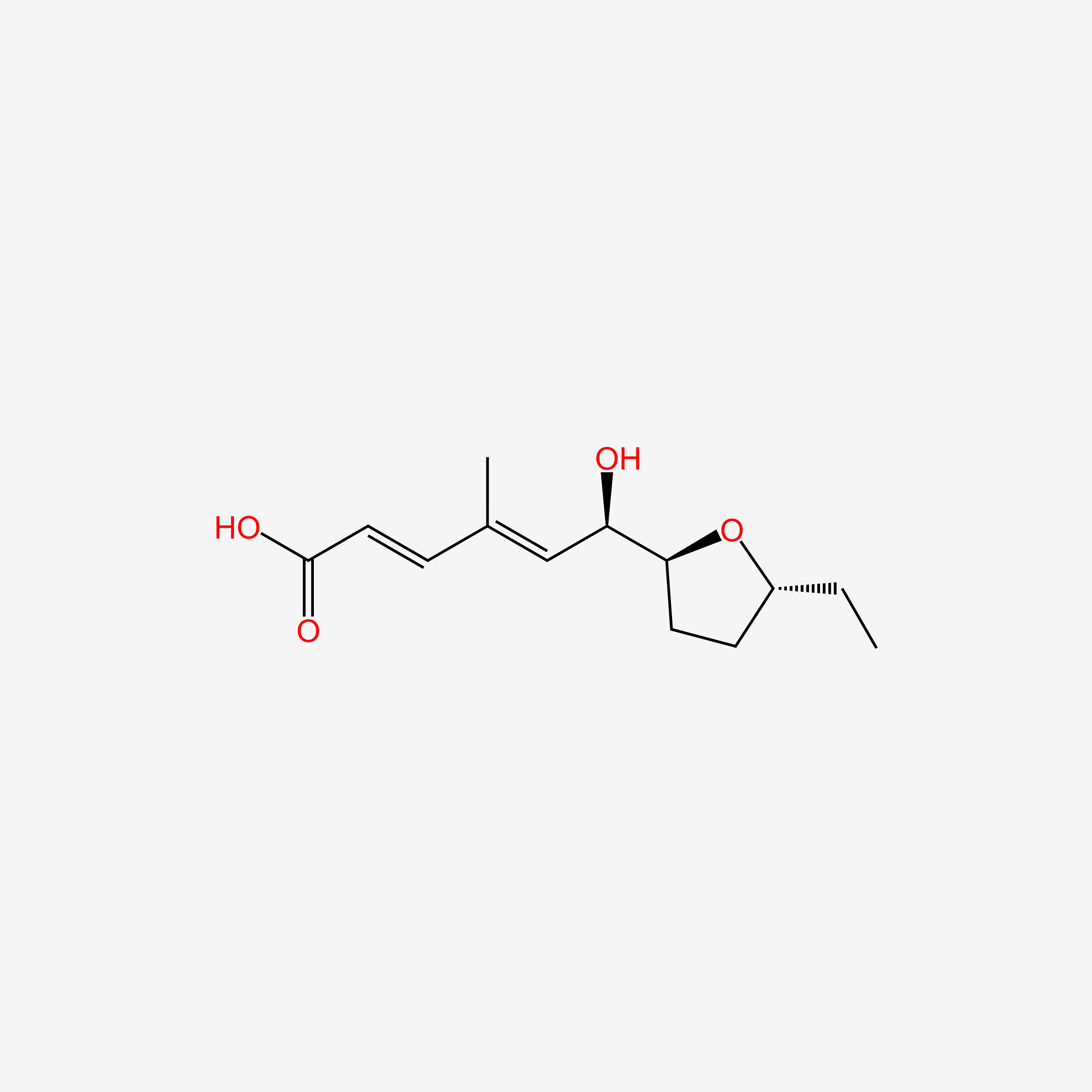 |
0.269 | D0V9EN |  |
0.250 | ||
| ENC006058 | 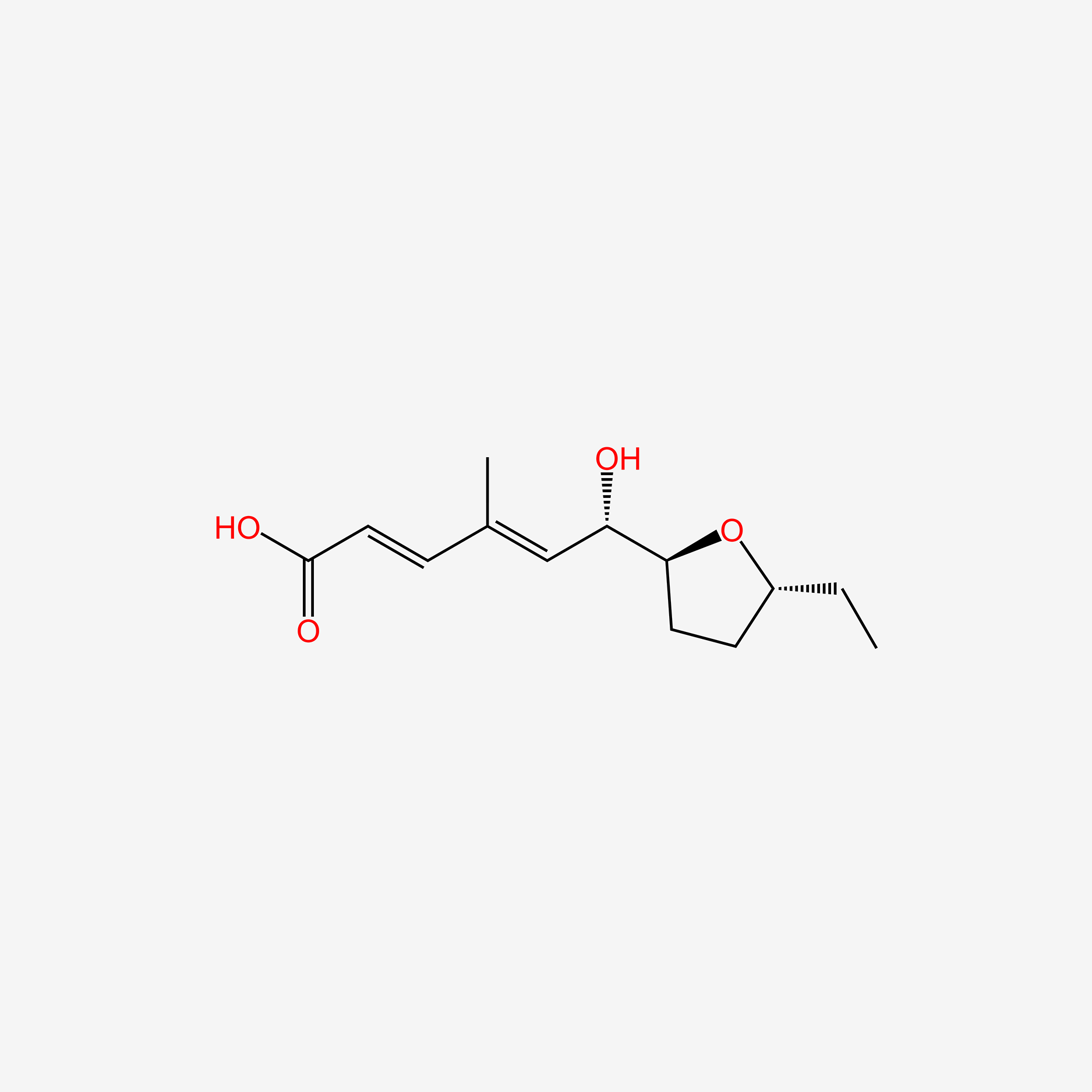 |
0.269 | D01NJI | 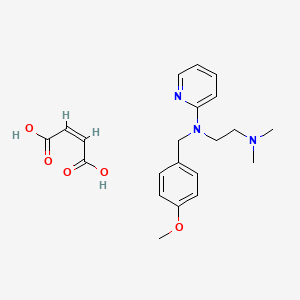 |
0.250 | ||