NPs Basic Information
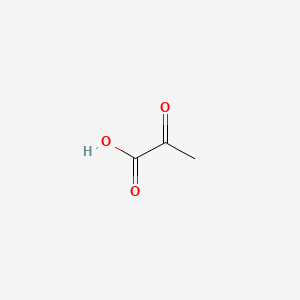
|
Name |
Pyruvic Acid
|
| Molecular Formula | C3H4O3 | |
| IUPAC Name* |
2-oxopropanoic acid
|
|
| SMILES |
CC(=O)C(=O)O
|
|
| InChI |
InChI=1S/C3H4O3/c1-2(4)3(5)6/h1H3,(H,5,6)
|
|
| InChIKey |
LCTONWCANYUPML-UHFFFAOYSA-N
|
|
| Synonyms |
Pyruvic acid; 2-Oxopropanoic acid; 127-17-3; acetylformic acid; Pyroracemic acid; 2-Oxopropionic acid; alpha-ketopropionic acid; 2-Ketopropionic acid; Propanoic acid, 2-oxo-; 2-Oxopropanoate; 2-oxo-propionic acid; 2-Oxopropansaeure; 2-Oxopropionsaeure; alpha-Oxopropionsaeure; Pyruvic acid (natural); acide pyruvique; a-Ketopropionic acid; Propanoic acid, oxo-; CH3COCOOH; FEMA No. 2970; alpha-keto propionic acid; NSC 179; .alpha.-Ketopropionic acid; 2-KETOPROPANOIC ACID; AI3-11220; 8558G7RUTR; CHEBI:32816; NSC-179; DSSTox_CID_1650; 2-oxo-Propanoic acid; DSSTox_RID_76263; DSSTox_GSID_21650; pyruvicacid; Brenztraubensaeure; CAS-127-17-3; Pyruvic acid (8CI); EINECS 204-824-3; BRN 0506211; UNII-8558G7RUTR; Acetylformate; Pyroracemate; a-Ketopropionate; 2-Oxopropionate; acetyl formic acid; 2-Oxopropanoicacid; alpha-Ketopropionate; 2-Oxopropanoic'acid; MFCD00002585; nchembio867-comp8; 2-oxo propanoic acid; Pyruvic acid, 95%; Pyruvic acid, 98%; 4b5s; bmse000112; PYRUVIC ACID [MI]; Pyruvic-2-[13C] acid; P-9250; NCIOpen2_000039; PYRUVIC ACID [FHFI]; PYRUVIC ACID [INCI]; 4-03-00-01505 (Beilstein Handbook Reference); Pyruvic acid, p.a., 98%; PYRUVIC ACID [WHO-DD]; Pyruvic acid, >=97%, FG; GTPL4809; NSC179; CHEMBL1162144; DTXSID2021650; BDBM19473; Pyruvic acid, natural, >=80%; AMY6082; Propanoic acid, 2-oxo- (9CI); HY-Y0781; ZINC1532517; Tox21_202096; Tox21_303284; BBL027390; LMFA01060077; s3143; 2-Oxopropanoic acid, Pyroracemic acid; AKOS000118803; CS-W020190; DB00119; NCGC00165990-01; NCGC00165990-02; NCGC00257000-01; NCGC00259645-01; Pyruvic acid, technical, >=95.0% (T); FT-0612738; P0579; EN300-21306; C00022; Q213580; B3CFF0AD-2F35-484C-B062-4AC88A1B2830; F2191-0254; Z104495240; Pyruvic acid, United States Pharmacopeia (USP) Reference Standard
|
|
| CAS | 127-17-3 | |
| PubChem CID | 1060 | |
| ChEMBL ID | CHEMBL1162144 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 88.06 | ALogp: | -0.3 |
| HBD: | 1 | HBA: | 3 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 54.4 | Aromatic Rings: | 0 |
| Heavy Atoms: | 6 | QED Weighted: | 0.462 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.232 | MDCK Permeability: | 0.00055729 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.026 | 20% Bioavailability (F20%): | 0.026 |
| 30% Bioavailability (F30%): | 0.145 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.325 | Plasma Protein Binding (PPB): | 22.31% |
| Volume Distribution (VD): | 0.208 | Fu: | 64.73% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.03 | CYP1A2-substrate: | 0.095 |
| CYP2C19-inhibitor: | 0.034 | CYP2C19-substrate: | 0.056 |
| CYP2C9-inhibitor: | 0.013 | CYP2C9-substrate: | 0.354 |
| CYP2D6-inhibitor: | 0.014 | CYP2D6-substrate: | 0.182 |
| CYP3A4-inhibitor: | 0.013 | CYP3A4-substrate: | 0.065 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 3.077 | Half-life (T1/2): | 0.859 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.018 | Human Hepatotoxicity (H-HT): | 0.118 |
| Drug-inuced Liver Injury (DILI): | 0.266 | AMES Toxicity: | 0.036 |
| Rat Oral Acute Toxicity: | 0.032 | Maximum Recommended Daily Dose: | 0.005 |
| Skin Sensitization: | 0.653 | Carcinogencity: | 0.022 |
| Eye Corrosion: | 0.989 | Eye Irritation: | 0.996 |
| Respiratory Toxicity: | 0.06 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000009 | 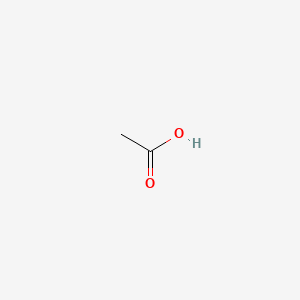 |
0.500 | D0G4JI | 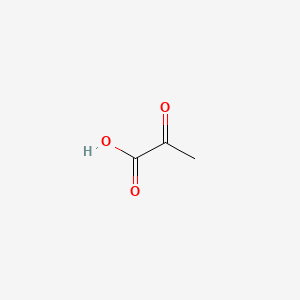 |
1.000 | ||
| ENC000403 | 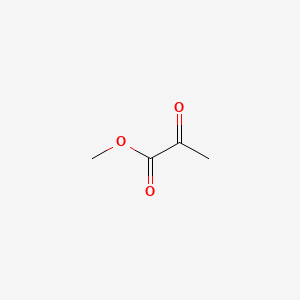 |
0.429 | D04CRL | 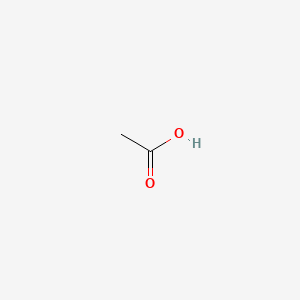 |
0.500 | ||
| ENC000879 | 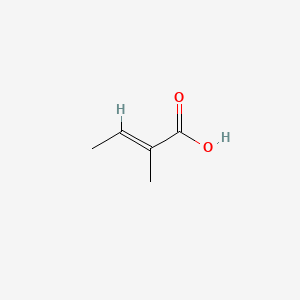 |
0.429 | D0F1GS | 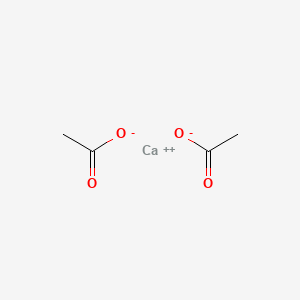 |
0.364 | ||
| ENC000001 | 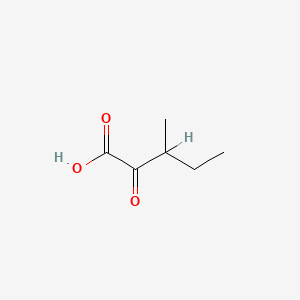 |
0.400 | D0Z4NI | 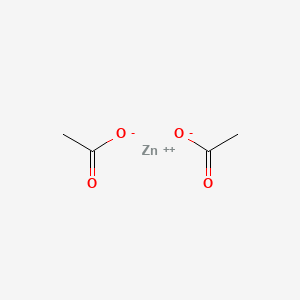 |
0.364 | ||
| ENC000410 | 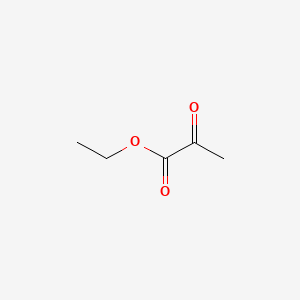 |
0.375 | D06XGW | 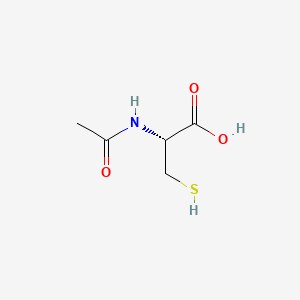 |
0.357 | ||
| ENC000418 | 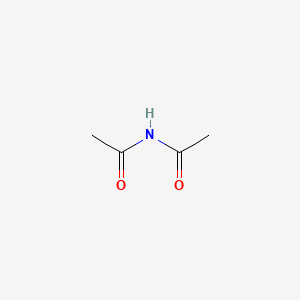 |
0.364 | D0R9BG | 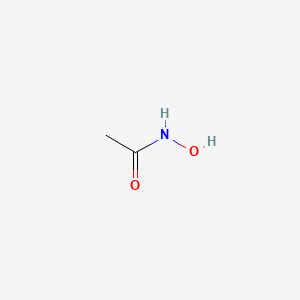 |
0.333 | ||
| ENC005488 | 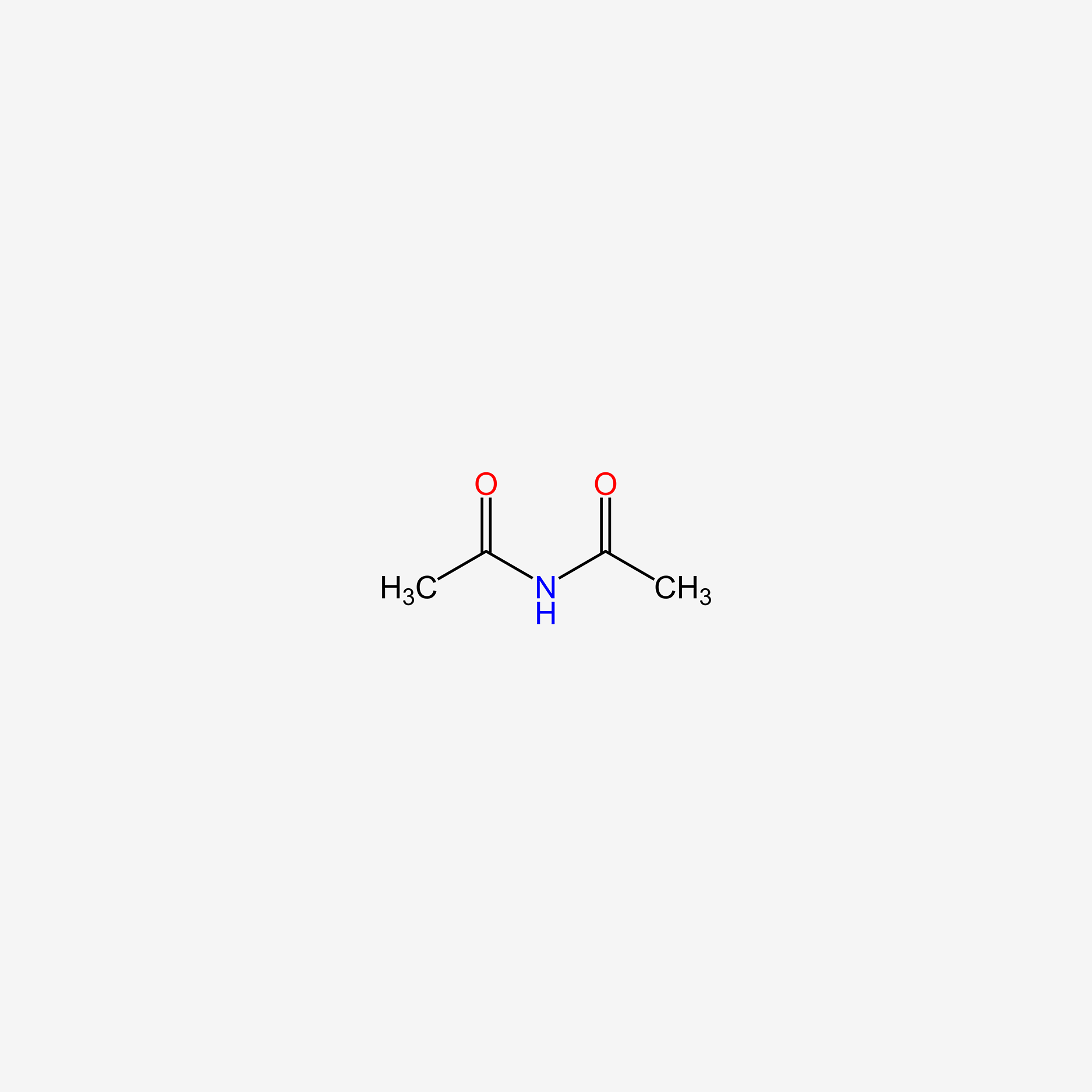 |
0.364 | D06VNK | 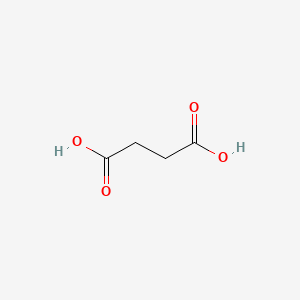 |
0.320 | ||
| ENC002070 | 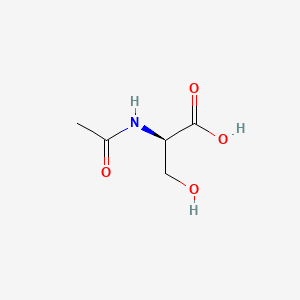 |
0.357 | D0A8CJ | 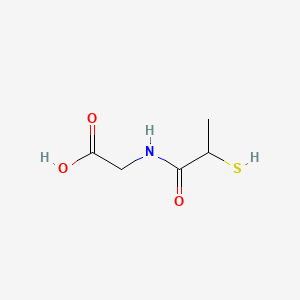 |
0.310 | ||
| ENC000044 | 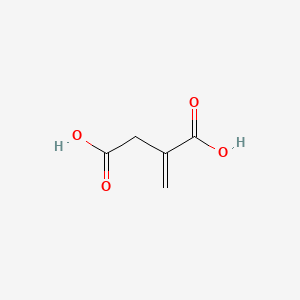 |
0.346 | D09PUL | 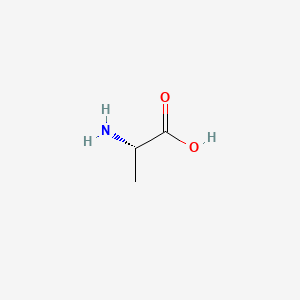 |
0.300 | ||
| ENC000288 | 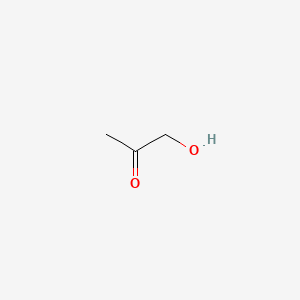 |
0.333 | D0Z4UY | 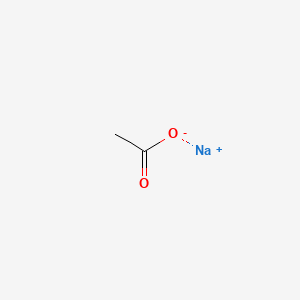 |
0.294 | ||