NPs Basic Information
|
Name |
Wortmannin
|
| Molecular Formula | C23H24O8 | |
| IUPAC Name* |
[(1R,3R,5S,9R,18S)-18-(methoxymethyl)-1,5-dimethyl-6,11,16-trioxo-13,17-dioxapentacyclo[10.6.1.02,10.05,9.015,19]nonadeca-2(10),12(19),14-trien-3-yl] acetate
|
|
| SMILES |
CC(=O)O[C@@H]1C[C@]2([C@@H](CCC2=O)C3=C1[C@]4([C@H](OC(=O)C5=COC(=C54)C3=O)COC)C)C
|
|
| InChI |
InChI=1S/C23H24O8/c1-10(24)30-13-7-22(2)12(5-6-14(22)25)16-18(13)23(3)15(9-28-4)31-21(27)11-8-29-20(17(11)23)19(16)26/h8,12-13,15H,5-7,9H2,1-4H3/t12-,13+,15+,22-,23-/m0/s1
|
|
| InChIKey |
QDLHCMPXEPAAMD-QAIWCSMKSA-N
|
|
| Synonyms |
wortmannin; 19545-26-7; Wartmannin; KY 12420; SL-2052; Antibiotic SL-2052; NSC221019; XVA4O219QW; KY-12420; MLS002703028; CHEMBL428496; CHEBI:52289; NSC-627609; Pi 3-Kinase Inhibitor; (1S,6bR,9aS,11R,11bR)-1-(methoxymethyl)-9a,11b-dimethyl-3,6,9-trioxo-1,6,6b,7,8,9,9a,10,11,11b-decahydro-3H-furo[4,3,2-de]indeno[4,5-h]isochromen-11-yl acetate; (1alpha,11alpha)-11-(Acetyloxy)-1-(methoxymethyl)-2-oxaandrosta-5,8-dieno(6,5,4-bc)furan-3,7,17-trione; (1R,3R,5S,9R,18S)-18-(methoxymethyl)-1,5-dimethyl-6,11,16-trioxo-13,17-dioxapentacyclo[10.6.1.0^{2,10}.0^{5,9}.0^{15,19}]nonadeca-2(10),12(19),14-trien-3-yl acetate; (1S,6bR,9aS,11R,11bR)-1-(methoxymethyl)-9a,11b-dimethyl-3,6,9-trioxo-3,6,6b,7,8,9,9a,10,11,11b-decahydro-1H-furo[4,3,2-de]indeno[4,5-h]isochromen-11-yl acetate; (1S,6bR,9aS,11R,11bR)-1-(methoxymethyl)-9a,11b-dimethyl-3,6,9-trioxo-3,6,6b,7,8,9,9a,10,11,11b-decahydro-1H-furo[4,3,2-de]indeno[4,5-h]isochromen-11-yl acetate.; (1S,6bR,9aS,11R,11bR)-9a,11b-dimethyl-1-[(methyloxy)methyl]-3,6,9-trioxo-1,6,6b,7,8,9,9a,10,11,11b-decahydro-3H-furo[4,3,2-de]indeno[4,5-h]isochromen-11-yl acetate; MFCD00133927; UNII-XVA4O219QW; wortmanin; NSC627609; Wortmannin, Wm; 3H-FURO(4,3,2-DE)INDENO(4,5-H)-2-BENZOPYRAN-3,6,9-TRIONE, 11-(ACETYLOXY)-1,6B,7,8,9A,10,11,11B-OCTAHYDRO-1-(METHOXYMETHYL)-9A,11B-DIMETHYL-, (1S,6BR,9AS,11R,11BR)-; 3H-Furo[4,3,2-de]indeno[4,5-h]-2-benzopyran-3,6,9-trione, 11-(acetyloxy)-1,6b,7,8,9a,10,11,11b-octahydro-1-(methoxymethyl)-9a,11b-dimethyl-, (1S,6bR,9aS,11R,11bR)-; nchembio866-comp1; NSC 627609; BRN 0067676; WORTMANNIN [MI]; (+)-WORTMANNIN; SCHEMBL4531; BSPBio_001232; 4-19-00-03134 (Beilstein Handbook Reference); cid_312145; GTPL6060; MEGxm0_000446; DTXSID8040642; Wortmannin,Penicillium wortmannin; ACon0_000951; BDBM15234; HMS1792N13; HMS1990N13; HMS3403N13; Wortmannin, Penicillium funiculosum; EX-A1930; ZINC1619592; s2758; ST-415; Wortmannin - CAS 19545-26-7; CCG-208290; CS-5073; DB08059; KY12420; NSC-221019; Wortmannin, from Penicillium funiculosum; Wortmannin; SL-2052; KY-12420; NCGC00163485-01; NCGC00163485-02; AC-35862; BS-16306; HY-10197; NCI60_001835; SMR001566836; W0007; CU-00000000011-1; J-012661; BRD-K87343924-001-02-4; 3,2-DE]INDENO[4,5-H][2]BENZOPYRAN-11-YL ACETATE; Wortmannin, from Penicillium funiculosum, >=98% (HPLC and TLC); 3H-furo[4,3,2-de]indeno[4,5-h]-2-benzopyran-3,6,9-trione, 11-(acetyloxy)-1,; (1R,6bR,9aS,11R,11bR)-1-(methoxymethyl)-9a,11b-dimethyl-3,6,9-trioxo-1,6,6b,7,8,9,9a,10,11,11b- decahydro-3H-furo[4,3,2-de]indeno[4,5-h]isochromen-11-yl acetate; (1S,6bR,9aS,11R,11bR)-1-(methoxymethyl)-9a,11b-dimethyl-3,6,9-trioxo-1,6,6b,7,8,9,9a,10,11,11b-decahydro-3H-furo[4,3,2-de]indeno[4,5-h]isochromen-11-ylacetate; (1S,6BR,9AS,11R,11BR)-9A,11B-DIMETHYL-1-[(METHYLOXY)METHYL]-3,6,9-TRIOXO-1,6,6B,7,8,9,9A,10,11,11B-DECAHYDRO-3H-FURO[4,; [(1R,3R,5S,9R,18S)-18-(methoxymethyl)-1,5-dimethyl-6,11,16-trioxo-13,17-dioxapentacyclo[10.6.1.02,10.05,9.015,19]nonadeca-2(10),12(19),14-trien-3-yl] acetate; 2-Oxaandrosta-5,5,4-bc]furan-3,7,17-trione, 11-(acetyloxy)-1-(methoxymethyl)-, (1.alpha.,11.alpha.)-; 3H-Furo[4,2-de]indeno[4,5,-h]-2-benzopyran-3,6,9-trione, 11-(Acetyloxy)-1,6b,7,8,9a,10,11,11b-octahydro-1-(methoxymethyl)-9a,11b-dimethyl-, (1S,6br,9aS,11R,11bR); 3H-Furo[4,2-de]indeno[4,5-h]-2-benzopyran-3,6,9-trione, 11-(acetyloxy)-1,6b,7,8,9a,10,11,11b-octahydro-1-(methoxymethyl)-9a,11b-dimethyl-, (1S,6br,9aS,11R,11bR); 3H-Furo[4,3,2-de]indeno[4,5-h]-2-benzopyran-3,6,9-trione, 11-(acetyloxy)-1,6b,7,8,9a,10,11,11b-; 3H-Furo[4,3,2-de]indeno[4,5-h]-2-benzopyran-3,6,9-trione,11-(acetyloxy)-1,6b,7,8,9a,10,11,11b-octahydro-1-(methoxymethyl)-9a,11b-dimethyl-, (1S,6bR,9aS,11R,11bR)-; 6b,7,8,9a,10,11,11b-octahydro-1-(methoxymethyl)-9a,11b-dimethyl-, (1S,6bR,9aS,11R,11bR)-; octahydro-1-(methoxymethyl)-9a,11b-dimethyl-, [1S-(1alpha,6baalpha,9abeta,11alpha,11bbeta)]-
|
|
| CAS | 19545-26-7 | |
| PubChem CID | 312145 | |
| ChEMBL ID | CHEMBL428496 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 428.4 | ALogp: | 1.2 |
| HBD: | 0 | HBA: | 8 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 109.0 | Aromatic Rings: | 5 |
| Heavy Atoms: | 31 | QED Weighted: | 0.675 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.889 | MDCK Permeability: | 0.00005250 |
| Pgp-inhibitor: | 0.987 | Pgp-substrate: | 0.479 |
| Human Intestinal Absorption (HIA): | 0.012 | 20% Bioavailability (F20%): | 0.958 |
| 30% Bioavailability (F30%): | 0.981 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.781 | Plasma Protein Binding (PPB): | 84.34% |
| Volume Distribution (VD): | 1.185 | Fu: | 17.86% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.086 | CYP1A2-substrate: | 0.314 |
| CYP2C19-inhibitor: | 0.125 | CYP2C19-substrate: | 0.258 |
| CYP2C9-inhibitor: | 0.228 | CYP2C9-substrate: | 0.056 |
| CYP2D6-inhibitor: | 0.015 | CYP2D6-substrate: | 0.098 |
| CYP3A4-inhibitor: | 0.629 | CYP3A4-substrate: | 0.355 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 4.469 | Half-life (T1/2): | 0.484 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.004 | Human Hepatotoxicity (H-HT): | 0.263 |
| Drug-inuced Liver Injury (DILI): | 0.329 | AMES Toxicity: | 0.136 |
| Rat Oral Acute Toxicity: | 0.961 | Maximum Recommended Daily Dose: | 0.896 |
| Skin Sensitization: | 0.222 | Carcinogencity: | 0.94 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.03 |
| Respiratory Toxicity: | 0.726 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
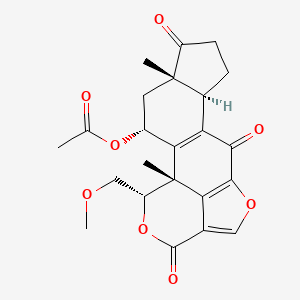
| ENC005378 | 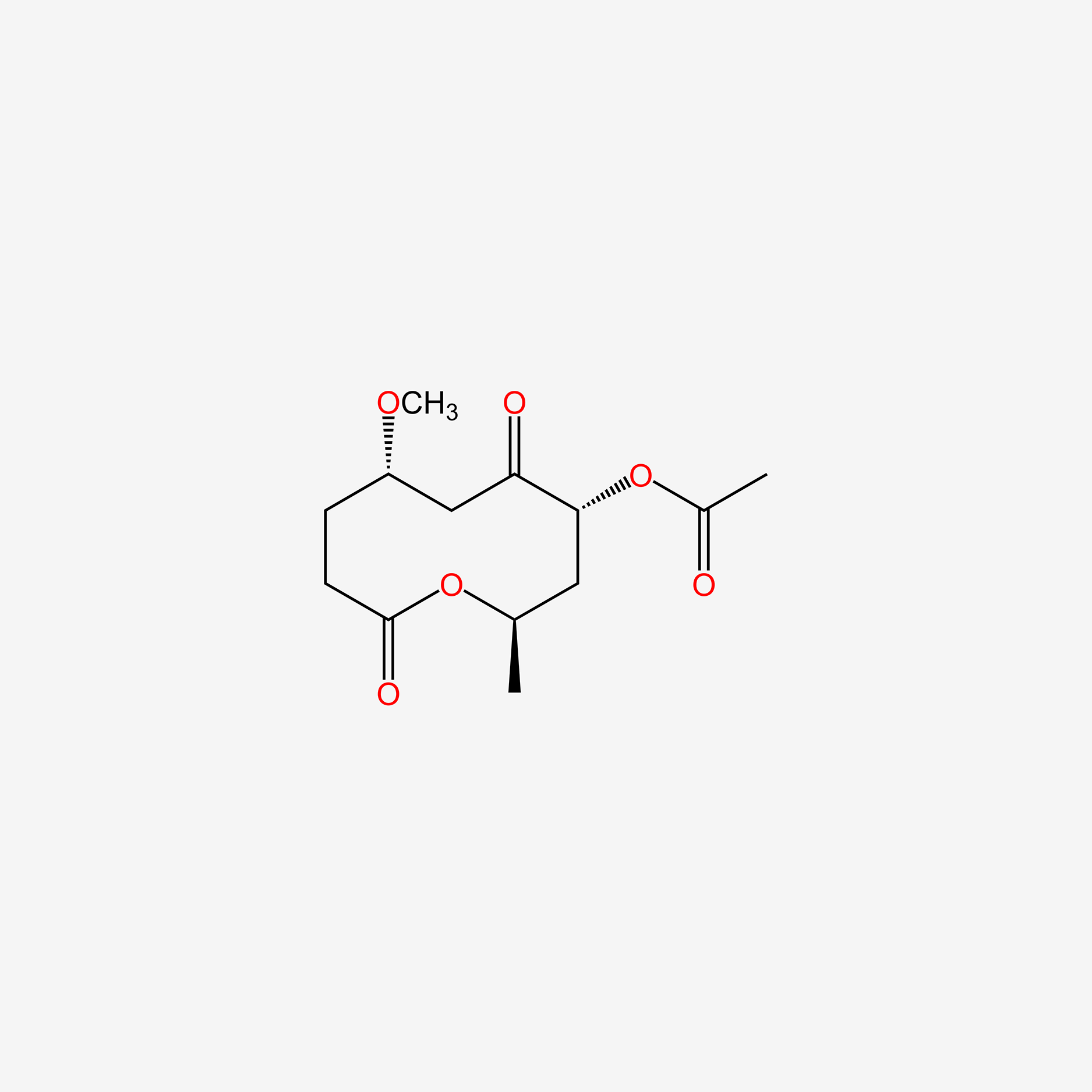 |
0.295 | D0X4RS | 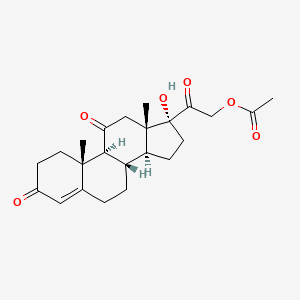 |
0.242 | ||
| ENC005151 | 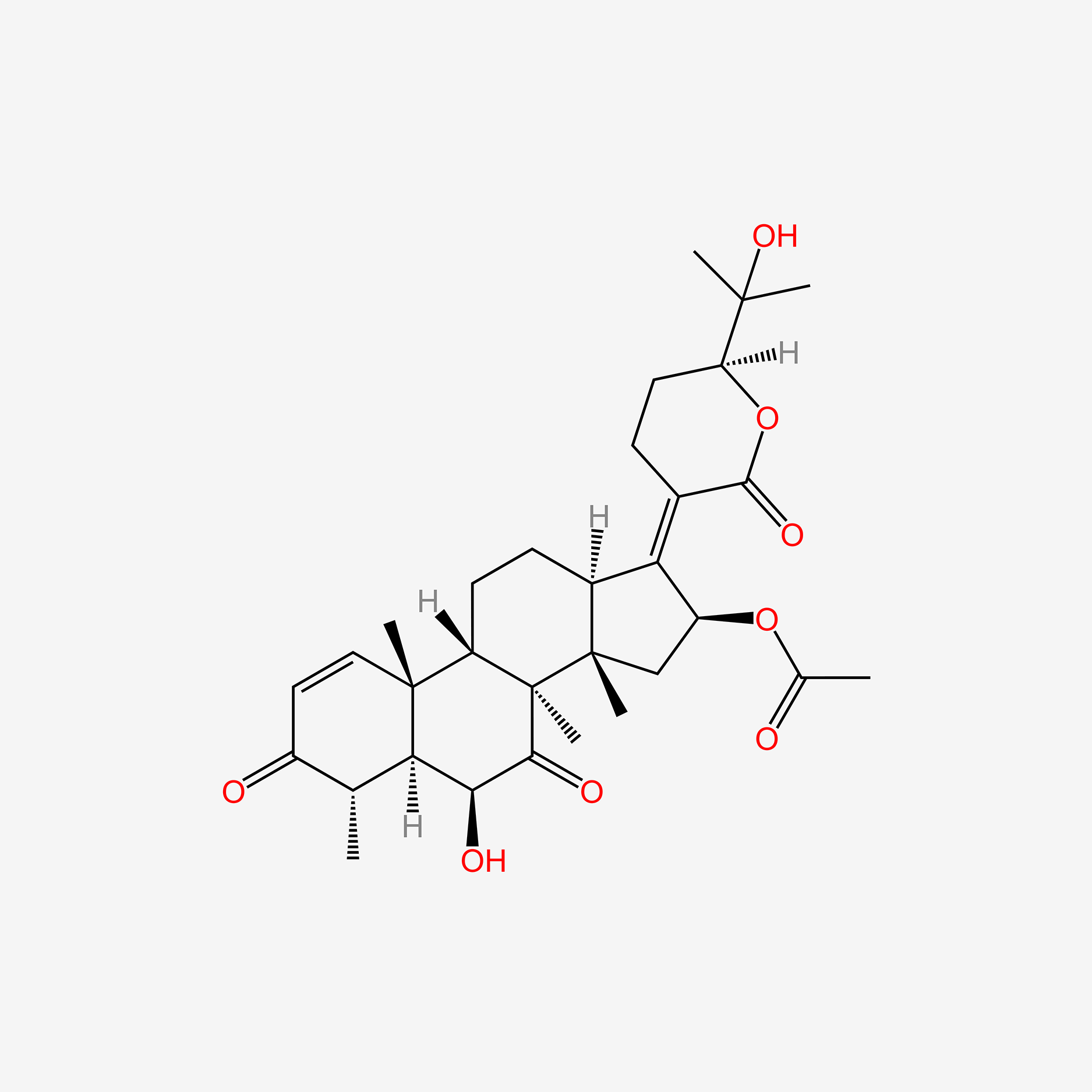 |
0.295 | D09WYX |  |
0.236 | ||
| ENC002386 |  |
0.288 | D01XWG |  |
0.229 | ||
| ENC003925 |  |
0.288 | D0D2VS | 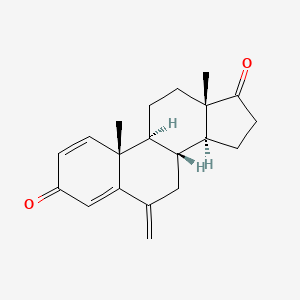 |
0.227 | ||
| ENC003846 | 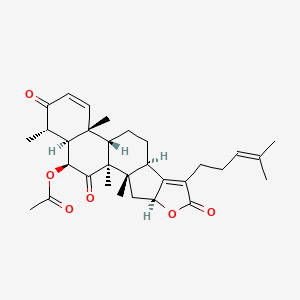 |
0.285 | D0Q4SD | 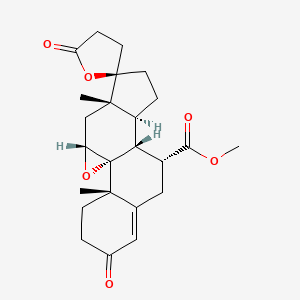 |
0.225 | ||
| ENC005031 |  |
0.277 | D0B9EJ | 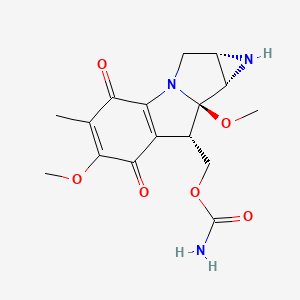 |
0.224 | ||
| ENC003138 | 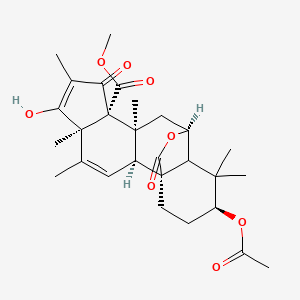 |
0.277 | D02CJX | 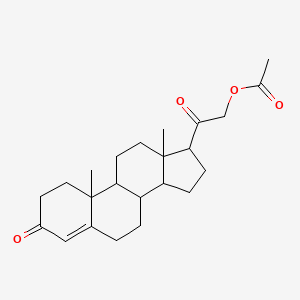 |
0.221 | ||
| ENC005318 | 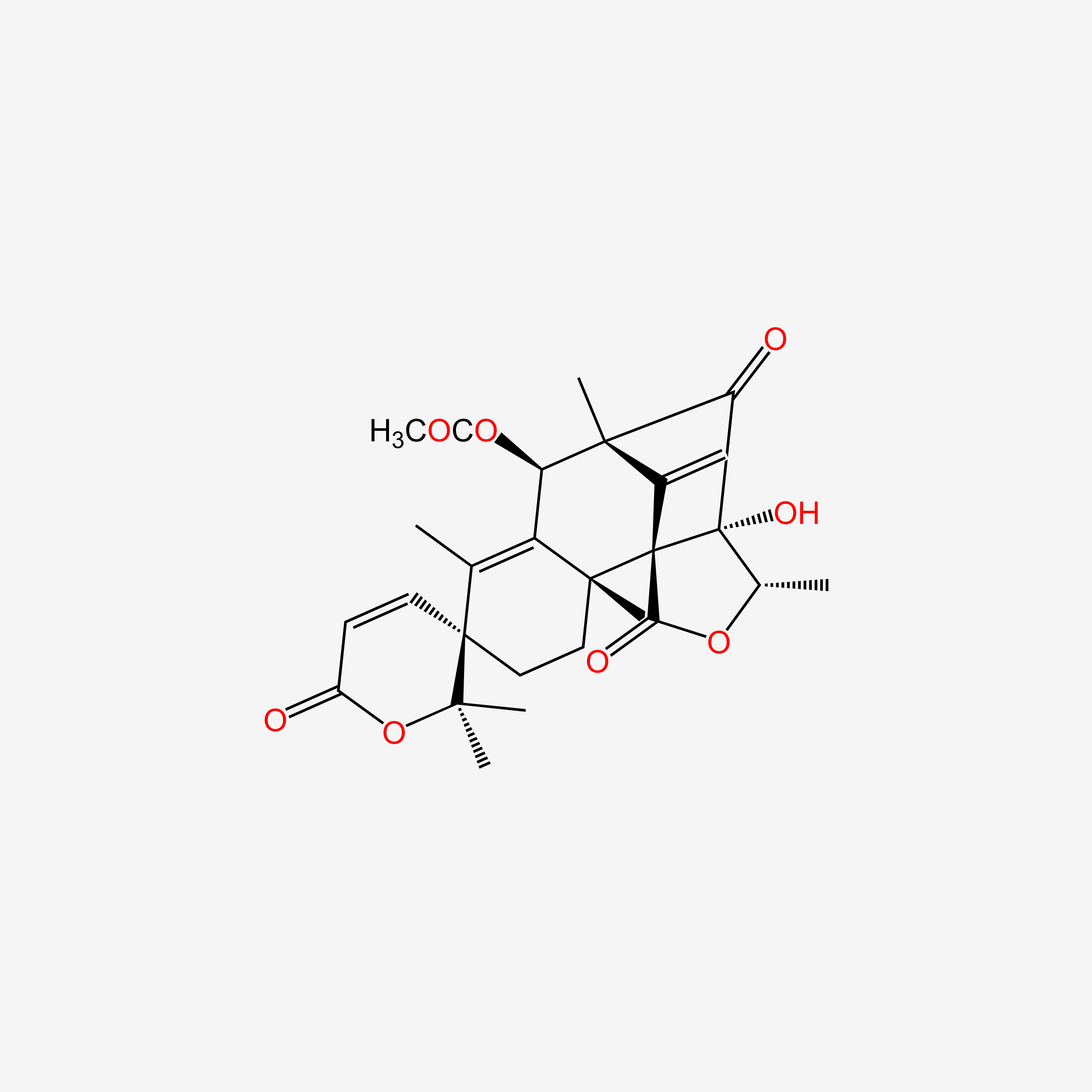 |
0.273 | D0Y0GH | 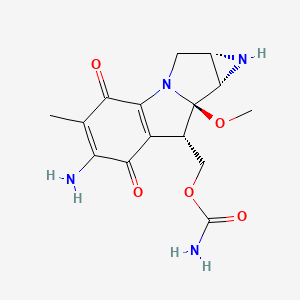 |
0.220 | ||
| ENC005188 | 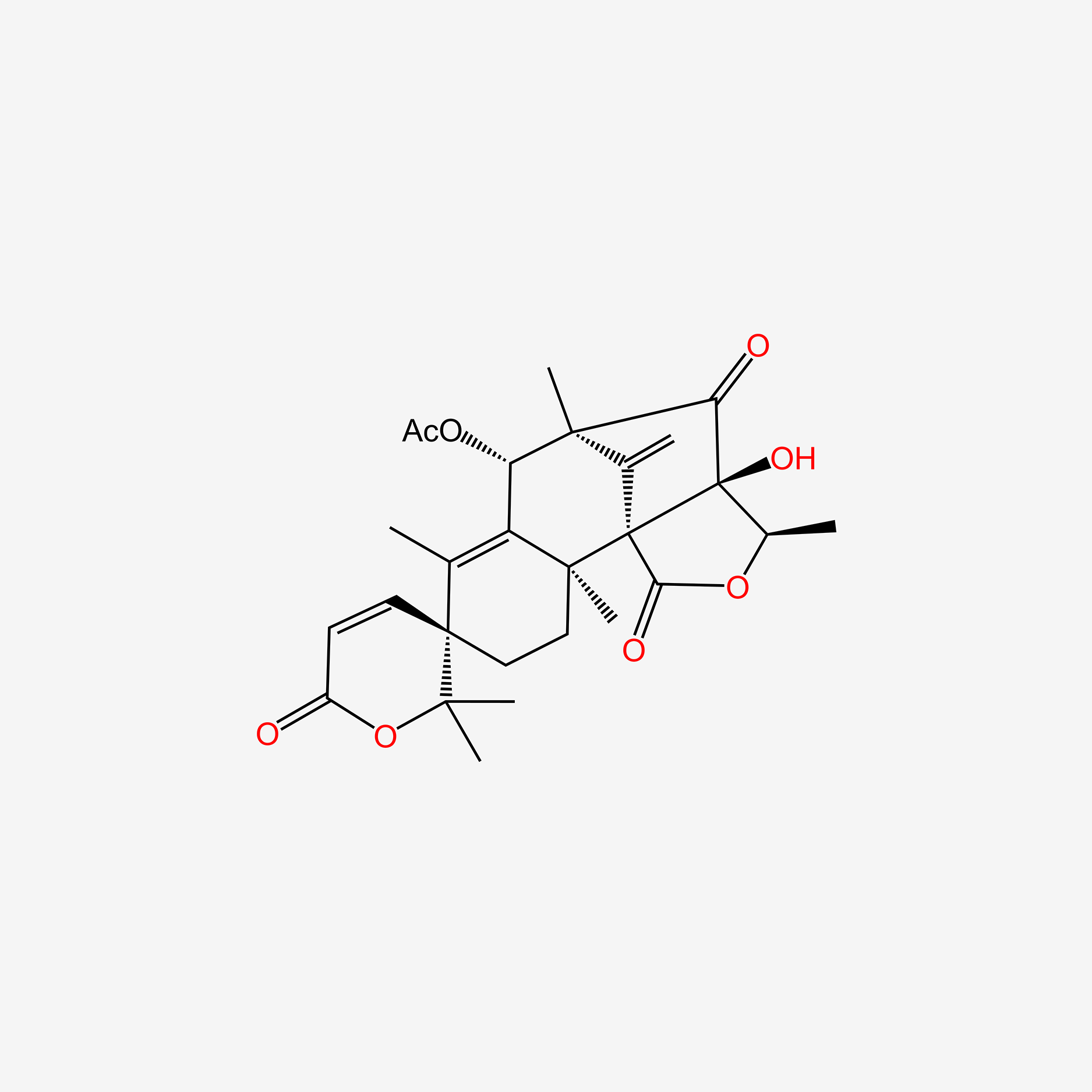 |
0.273 | D08BDT | 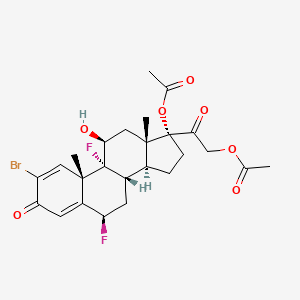 |
0.219 | ||
| ENC001980 | 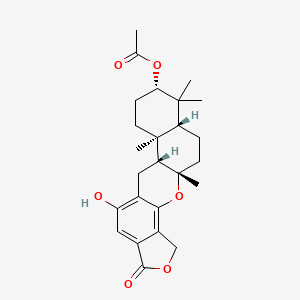 |
0.269 | D07VLY | 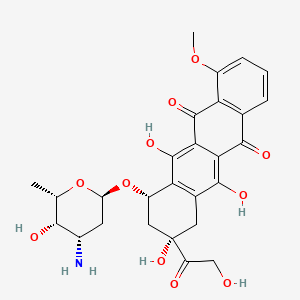 |
0.217 | ||