NPs Basic Information
|
Name |
(2,3,4,5,6-Pentaacetyloxycyclohexyl) acetate
|
| Molecular Formula | C18H24O12 | |
| IUPAC Name* |
(2,3,4,5,6-pentaacetyloxycyclohexyl) acetate
|
|
| SMILES |
CC(=O)OC1C(C(C(C(C1OC(=O)C)OC(=O)C)OC(=O)C)OC(=O)C)OC(=O)C
|
|
| InChI |
InChI=1S/C18H24O12/c1-7(19)25-13-14(26-8(2)20)16(28-10(4)22)18(30-12(6)24)17(29-11(5)23)15(13)27-9(3)21/h13-18H,1-6H3
|
|
| InChIKey |
SQUHHTBVTRBESD-UHFFFAOYSA-N
|
|
| Synonyms |
1254-38-2; myo-Inositol Hexaacetate; Myo-inositol, hexaacetate; (2,3,4,5,6-pentaacetyloxycyclohexyl) acetate; 1,2,3,4,5,6-Hexa-O-acetyl-myo-inositol; neo-Inositol hexaacetate; 18779-57-2; Cyclohexane-1,2,3,4,5,6-hexayl hexaacetate; 20097-40-9; 1-O,2-O,3-O,4-O,5-O,6-O-Hexaacetyl-muco-inositol; Myoinositol hexaacetate; (1R,2R,3S,4R,5s,6S)-cyclohexane-1,2,3,4,5,6-hexayl hexaacetate; 1-Hoami; Mesoinositol hexaacetate; Inositol, hexaacetate, myo-; Hexakis-O-acetyl-myo-inositol; inositol hexaacetate; 1,2,3,4,5,6-Hexaacetylinositol; cis-Inositol hexaacetate; epi-Inositol hexaacetate; allo-Inositol hexaacetate; muco-Inositol hexaacetate; scyllo-Inositol hexaacetate; D-chiro-Inositol hexaacetate; 29267-04-7; SCHEMBL1266094; SCHEMBL7151553; SCHEMBL7151917; SCHEMBL7152812; SCHEMBL7154470; SCHEMBL8329413; SCHEMBL8358880; SCHEMBL21510391; DTXSID40925122; NSC21068; NSC-21068; NSC232032; AKOS030241399; ZINC100052961; ZINC238778162; NSC-232032; 20108-71-8; 29307-62-8; CS-0443276; FT-0670352; 2,3,4,5,6-Pentakis(acetyloxy)cyclohexyl acetate #; W-200987; 1beta,2alpha,3beta,4beta,5beta,6alpha-Cyclohexanehexol hexaacetate; 20108-52-5; 29267-03-6
|
|
| CAS | 1254-38-2 | |
| PubChem CID | 228282 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 432.4 | ALogp: | -0.3 |
| HBD: | 0 | HBA: | 12 |
| Rotatable Bonds: | 12 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 158.0 | Aromatic Rings: | 1 |
| Heavy Atoms: | 30 | QED Weighted: | 0.41 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.21 | MDCK Permeability: | 0.00013017 |
| Pgp-inhibitor: | 0.966 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 1 | 20% Bioavailability (F20%): | 0.109 |
| 30% Bioavailability (F30%): | 0.994 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.104 | Plasma Protein Binding (PPB): | 24.73% |
| Volume Distribution (VD): | 0.799 | Fu: | 56.99% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.035 | CYP1A2-substrate: | 0.002 |
| CYP2C19-inhibitor: | 0.006 | CYP2C19-substrate: | 0.048 |
| CYP2C9-inhibitor: | 0 | CYP2C9-substrate: | 0.005 |
| CYP2D6-inhibitor: | 0.975 | CYP2D6-substrate: | 0.031 |
| CYP3A4-inhibitor: | 0.026 | CYP3A4-substrate: | 0.054 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 2.505 | Half-life (T1/2): | 0.516 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.003 | Human Hepatotoxicity (H-HT): | 0.616 |
| Drug-inuced Liver Injury (DILI): | 0.888 | AMES Toxicity: | 0.065 |
| Rat Oral Acute Toxicity: | 0.041 | Maximum Recommended Daily Dose: | 0.005 |
| Skin Sensitization: | 0.07 | Carcinogencity: | 0.042 |
| Eye Corrosion: | 0.915 | Eye Irritation: | 0.94 |
| Respiratory Toxicity: | 0.001 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
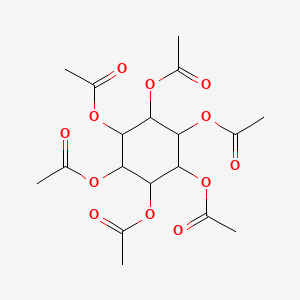
| ENC001032 | 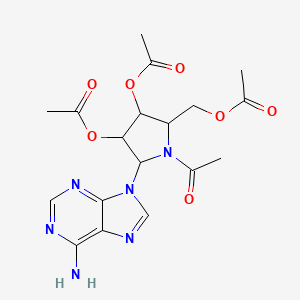 |
0.306 | D0L2UN | 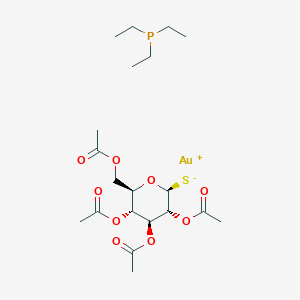 |
0.429 | ||
| ENC001010 |  |
0.286 | D0OL7F |  |
0.310 | ||
| ENC002259 |  |
0.254 | D09SIK |  |
0.310 | ||
| ENC003484 |  |
0.250 | D06IGU |  |
0.272 | ||
| ENC001480 |  |
0.247 | D0WP0B |  |
0.226 | ||
| ENC002467 |  |
0.247 | D0Q6DX | 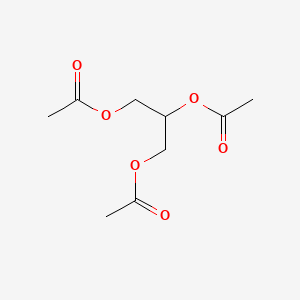 |
0.219 | ||
| ENC005487 | 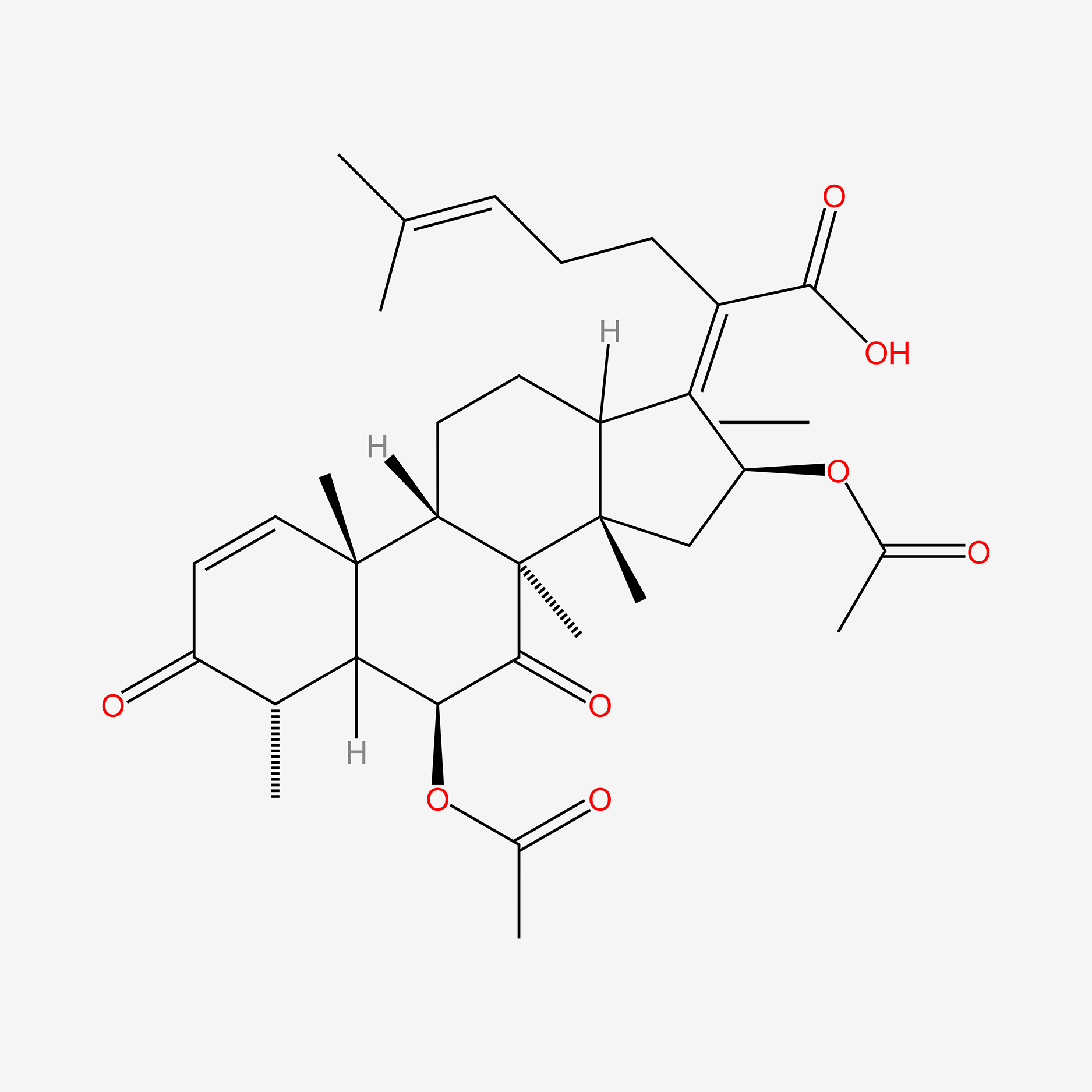 |
0.242 | D0C4RB | 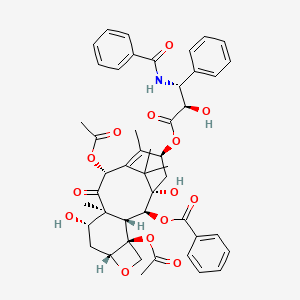 |
0.191 | ||
| ENC003485 | 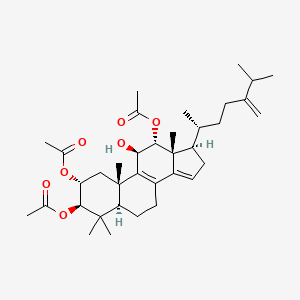 |
0.239 | D0J5TS | 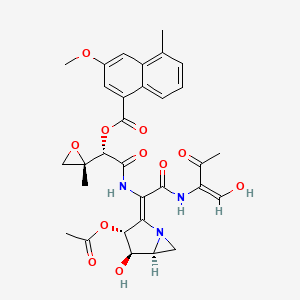 |
0.190 | ||
| ENC006083 | 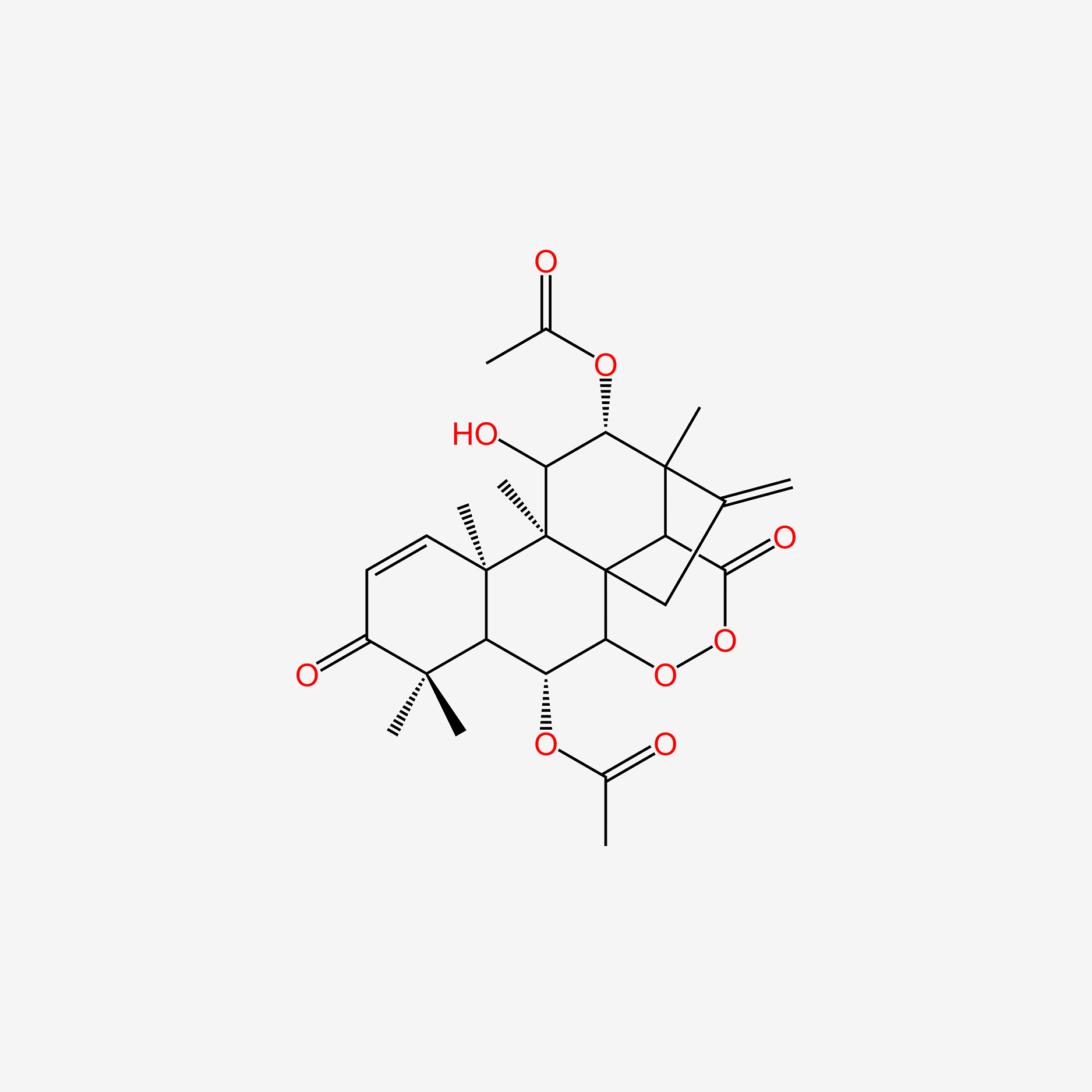 |
0.227 | D0X7XG | 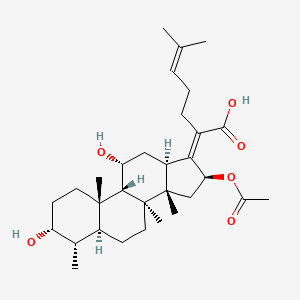 |
0.180 | ||
| ENC003104 | 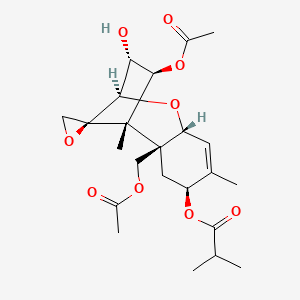 |
0.226 | D0G7KJ | 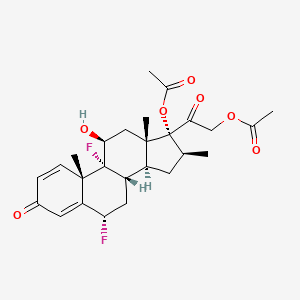 |
0.179 | ||