NPs Basic Information
|
Name |
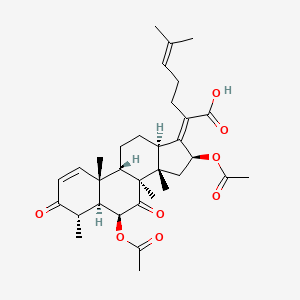
Helvolic acid
|
| Molecular Formula | C33H44O8 | |
| IUPAC Name* |
(2Z)-2-[(4S,5S,6S,8S,9S,10R,13R,14S,16S)-6,16-diacetyloxy-4,8,10,14-tetramethyl-3,7-dioxo-5,6,9,11,12,13,15,16-octahydro-4H-cyclopenta[a]phenanthren-17-ylidene]-6-methylhept-5-enoic acid
|
|
| SMILES |
C[C@H]1[C@@H]2[C@@H](C(=O)[C@]3([C@H]([C@]2(C=CC1=O)C)CC[C@@H]\4[C@@]3(C[C@@H](/C4=C(/CCC=C(C)C)\C(=O)O)OC(=O)C)C)C)OC(=O)C
|
|
| InChI |
InChI=1S/C33H44O8/c1-17(2)10-9-11-21(30(38)39)26-22-12-13-25-31(6)15-14-23(36)18(3)27(31)28(41-20(5)35)29(37)33(25,8)32(22,7)16-24(26)40-19(4)34/h10,14-15,18,22,24-25,27-28H,9,11-13,16H2,1-8H3,(H,38,39)/b26-21-/t18-,22+,24+,25+,27-,28+,31-,32+,33-/m1/s1
|
|
| InChIKey |
MDFZYGLOIJNNRM-OAJDADRGSA-N
|
|
| Synonyms |
helvolic acid; Fumigacin; 29400-42-8; MZX54GS8AH; CHEBI:62460; (2Z)-2-[(4S,5S,6S,8S,9S,10R,13R,14S,16S)-6,16-diacetyloxy-4,8,10,14-tetramethyl-3,7-dioxo-5,6,9,11,12,13,15,16-octahydro-4H-cyclopenta[a]phenanthren-17-ylidene]-6-methylhept-5-enoic acid; 16-(Acetyloxy)-3,7-dioxo-29-nordammara-1,17(20),24-trien-21-oic acid; HELVOLIC ACID FROM CEPHALOSPORIUM*CAERULENS; (4alpha,6beta,8alpha,9beta,13alpha,14beta,16beta,17beta)-6,16- bis(acetyloxy)-3,7-dioxo-29-nordammara-1,17(20),24-trien-21-oic acid; UNII-MZX54GS8AH; NSC 319943; BRN 3230584; NSC-319943; HELVOLIC ACID [MI]; 3-10-00-04777 (Beilstein Handbook Reference); CHEMBL505132; MEGxm0_000011; ACon0_000265; ACon1_000380; DTXSID201017874; HY-N6728; ZINC253530014; LMPR0106080005; NCGC00169132-01; NCGC00169132-02; 29-Nordammara-1,17(20),24-trien-21-oic acid, 6,16-bis(acetyloxy)-3,7-dioxo-, (4-alpha,6-beta,8-alpha,9-beta,13-alpha,14-beta,16-beta,17-beta)-; 29-Nordammara-1,17(20),24-trien-21-oic acid, 6,16-bis(acetyloxy)-3,7-dioxo-, (4.alpha.,6.beta.,8.alpha.,9.beta.,13.alpha.,14.beta.,16.beta.,17Z)-; BH162732; CS-0086553; J-017496; BRD-K08133434-001-01-0; Q27131924; (17Z)-6beta,16beta-diacetoxy-4alpha,8,14-trimethyl-3,7-dioxo-5alpha,8alpha,9beta,13alpha,14beta-18-norcholesta-1,17,24-trien-21-oic acid; (2Z)-2-(6,16-diacetyloxy-4,8,10,14-tetramethyl-3,7-dioxo-5,6,9,11,12,13,15,16-octahydro-4H-cyclopenta[a]phenanthren-17-ylidene)-6-methylhept-5-enoic acid; (2Z)-2-[(4alpha,5alpha,6beta,8alpha,9beta,13alpha,14beta,16beta,17Z)-6,16-diacetoxy-4,8,10,14-tetramethyl-3,7-dioxogon-1-en-17-ylidene]-6-methylhept-5-enoic acid; (Z)-6.BETA.,16.BETA.-DIHYDROXY-3,7-DIOXO-29-NOR-8.ALPHA.,9.BETA.,13.ALPHA.,14.BETA.-DAMMARA-1,17(20),24-TRIEN-21-OIC ACID DIACETATE; NCGC00169132-02_C33H44O8_(4alpha,5alpha,6beta,8alpha,9beta,13alpha,14beta,16beta,17Z)-6,16-Diacetoxy-4,8,14-trimethyl-3,7-dioxo-18-norcholesta-1,17,24-trien-21-oic acid
|
|
| CAS | 29400-42-8 | |
| PubChem CID | 3002143 | |
| ChEMBL ID | CHEMBL505132 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 568.7 | ALogp: | 5.1 |
| HBD: | 1 | HBA: | 8 |
| Rotatable Bonds: | 8 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 124.0 | Aromatic Rings: | 4 |
| Heavy Atoms: | 41 | QED Weighted: | 0.248 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.405 | MDCK Permeability: | 0.00004270 |
| Pgp-inhibitor: | 0.979 | Pgp-substrate: | 0.982 |
| Human Intestinal Absorption (HIA): | 0.2 | 20% Bioavailability (F20%): | 0.717 |
| 30% Bioavailability (F30%): | 0.739 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.969 | Plasma Protein Binding (PPB): | 95.54% |
| Volume Distribution (VD): | 0.563 | Fu: | 8.37% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.008 | CYP1A2-substrate: | 0.068 |
| CYP2C19-inhibitor: | 0.016 | CYP2C19-substrate: | 0.357 |
| CYP2C9-inhibitor: | 0.013 | CYP2C9-substrate: | 0.048 |
| CYP2D6-inhibitor: | 0.002 | CYP2D6-substrate: | 0.026 |
| CYP3A4-inhibitor: | 0.397 | CYP3A4-substrate: | 0.336 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 1.799 | Half-life (T1/2): | 0.219 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.001 | Human Hepatotoxicity (H-HT): | 0.196 |
| Drug-inuced Liver Injury (DILI): | 0.575 | AMES Toxicity: | 0.007 |
| Rat Oral Acute Toxicity: | 0.933 | Maximum Recommended Daily Dose: | 0.436 |
| Skin Sensitization: | 0.137 | Carcinogencity: | 0.7 |
| Eye Corrosion: | 0.934 | Eye Irritation: | 0.137 |
| Respiratory Toxicity: | 0.983 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC005487 | 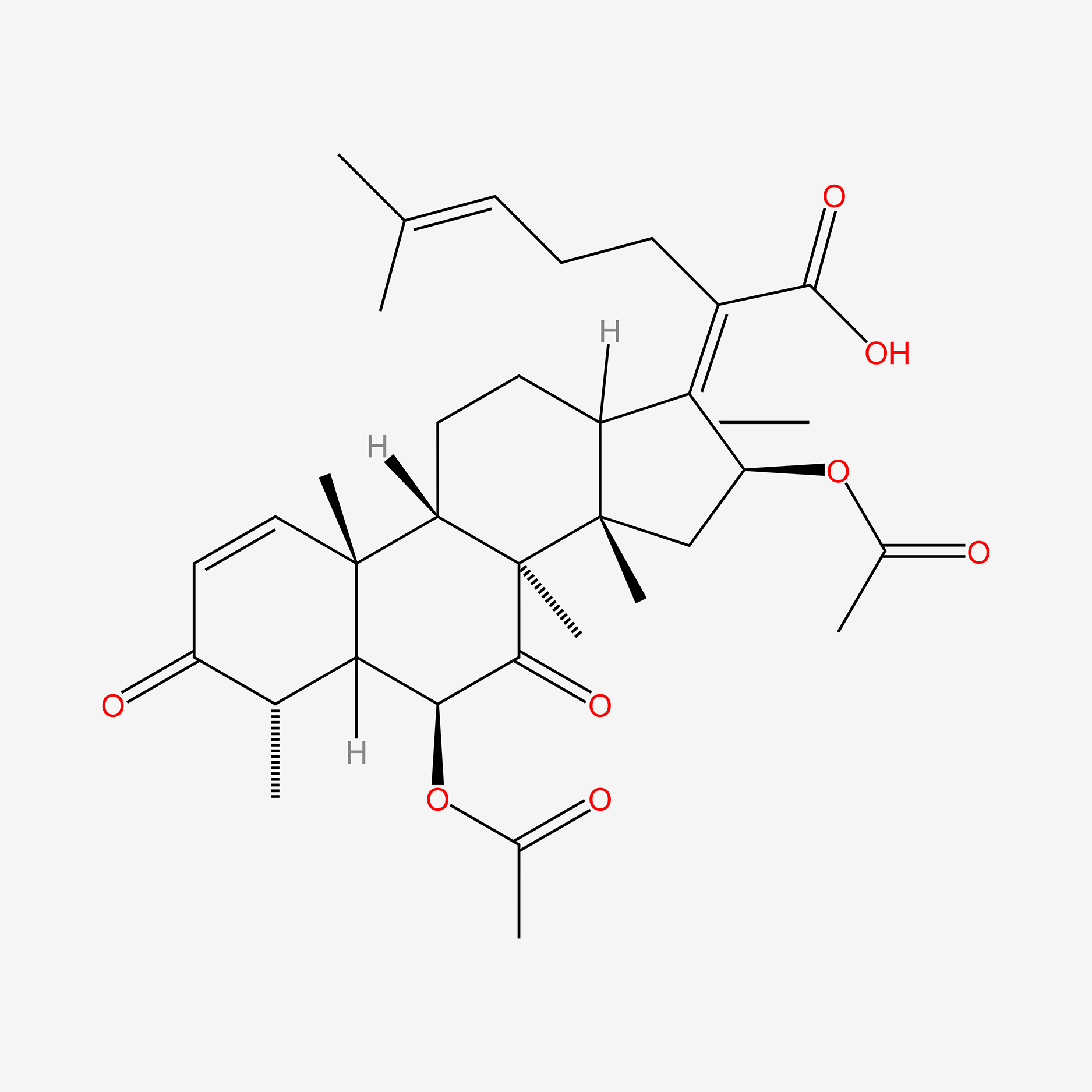 |
0.973 | D0X7XG | 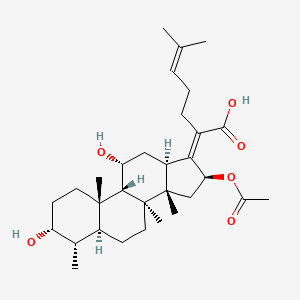 |
0.475 | ||
| ENC003484 | 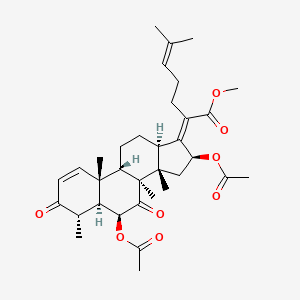 |
0.889 | D0G7KJ | 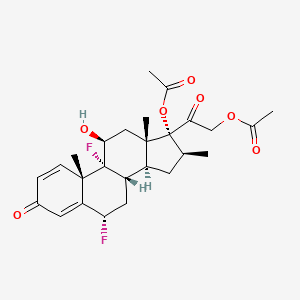 |
0.295 | ||
| ENC003780 | 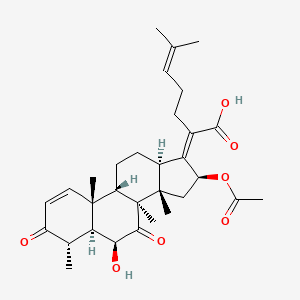 |
0.826 | D01ZOG | 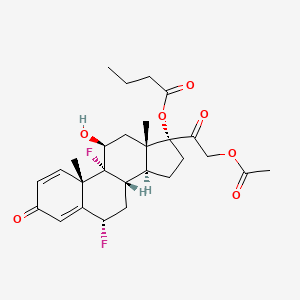 |
0.272 | ||
| ENC005154 | 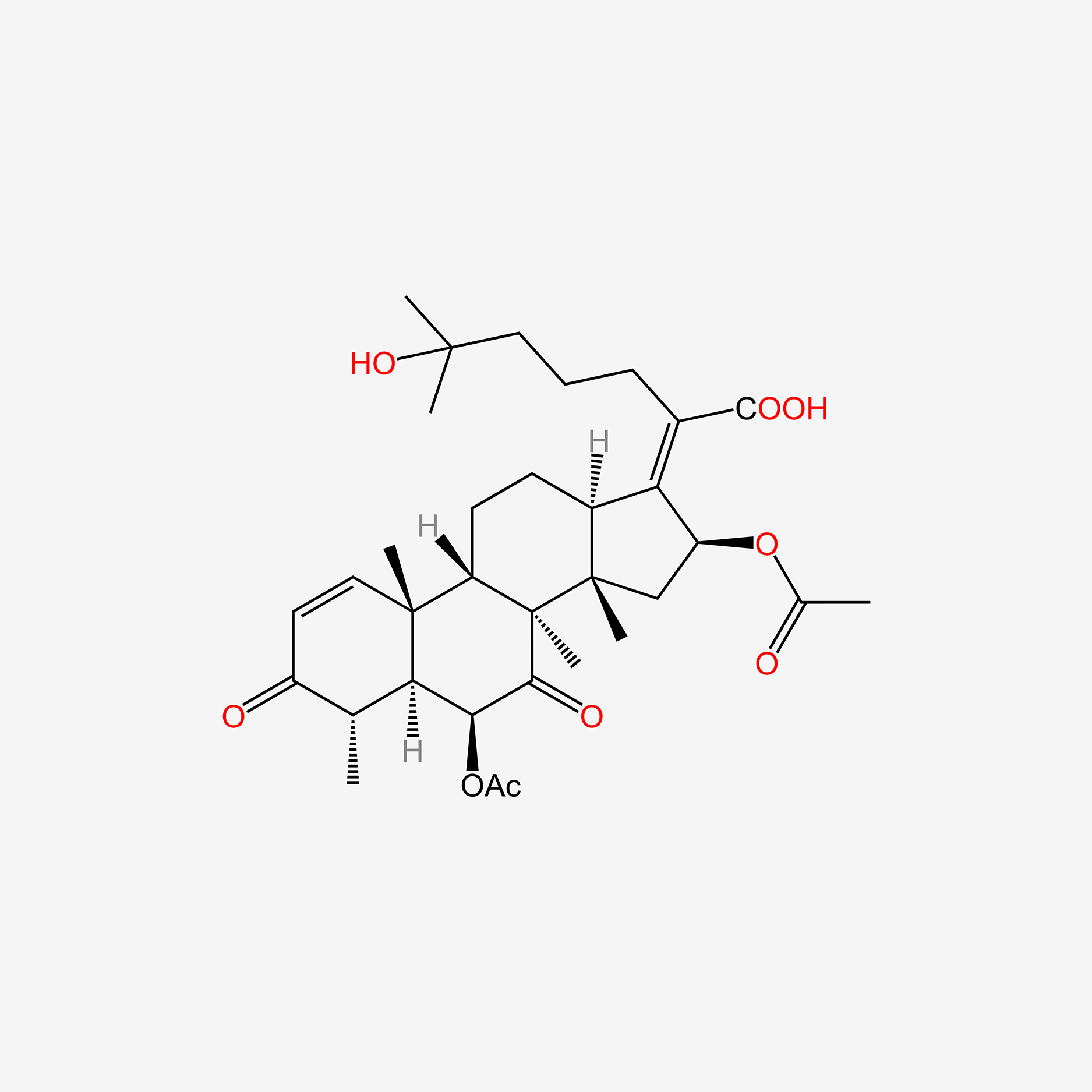 |
0.803 | D08BDT | 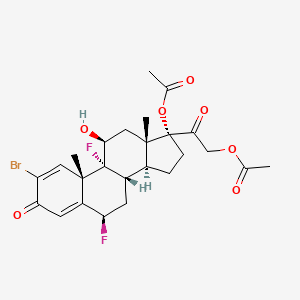 |
0.270 | ||
| ENC005236 | 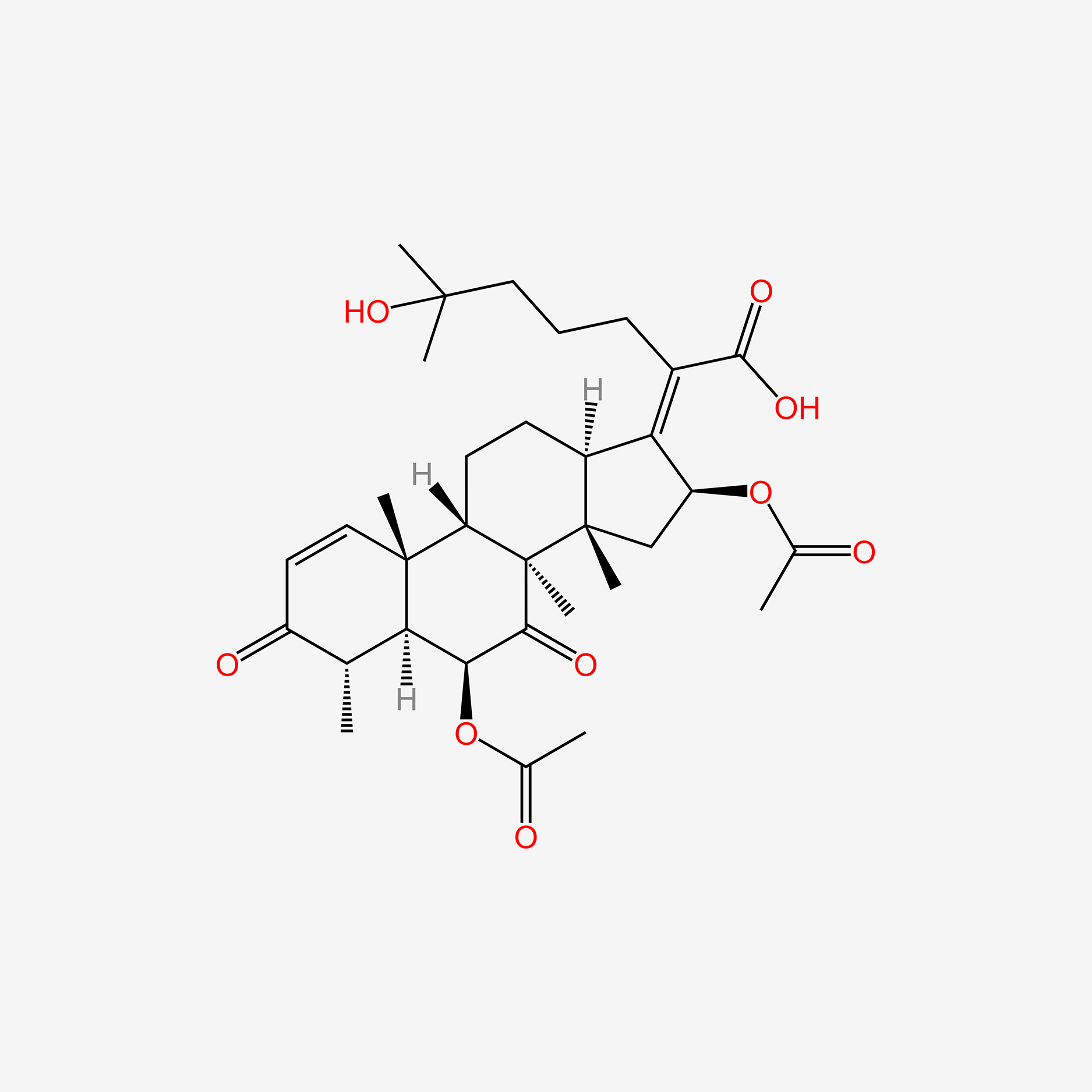 |
0.803 | D0H2MO | 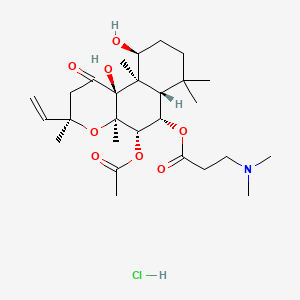 |
0.267 | ||
| ENC002467 | 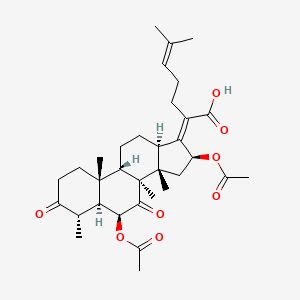 |
0.787 | D0X2LV | 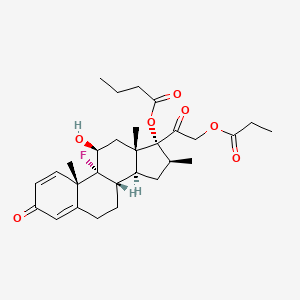 |
0.267 | ||
| ENC005155 | 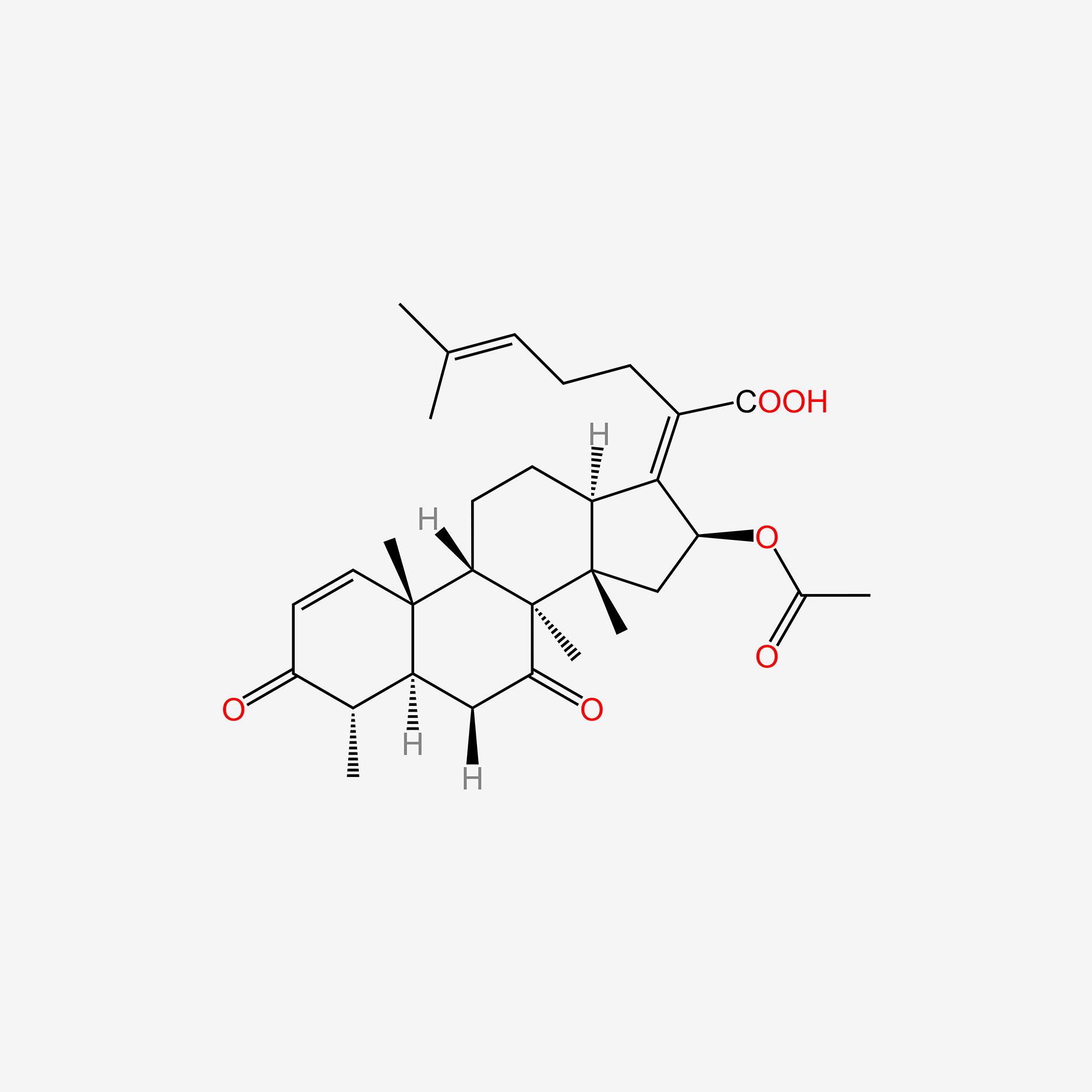 |
0.748 | D09IEE | 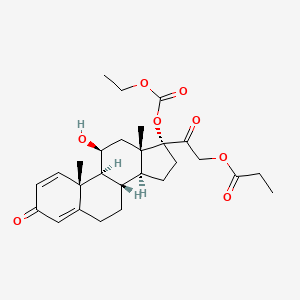 |
0.265 | ||
| ENC003848 | 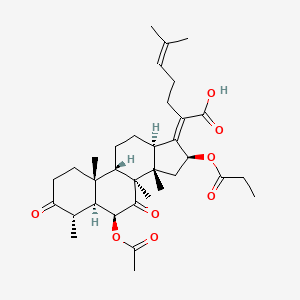 |
0.713 | D09WYX | 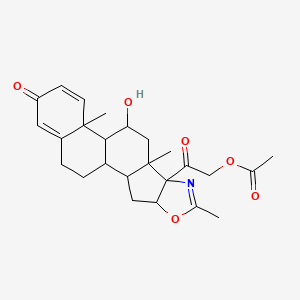 |
0.263 | ||
| ENC003846 | 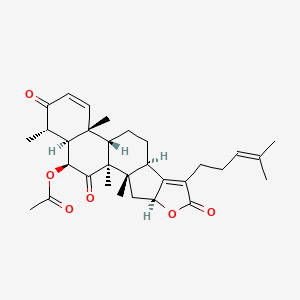 |
0.633 | D0D2TN | 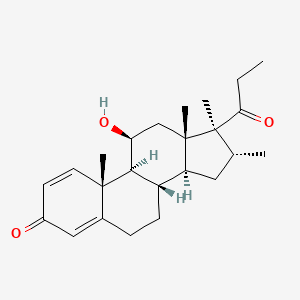 |
0.255 | ||
| ENC005153 | 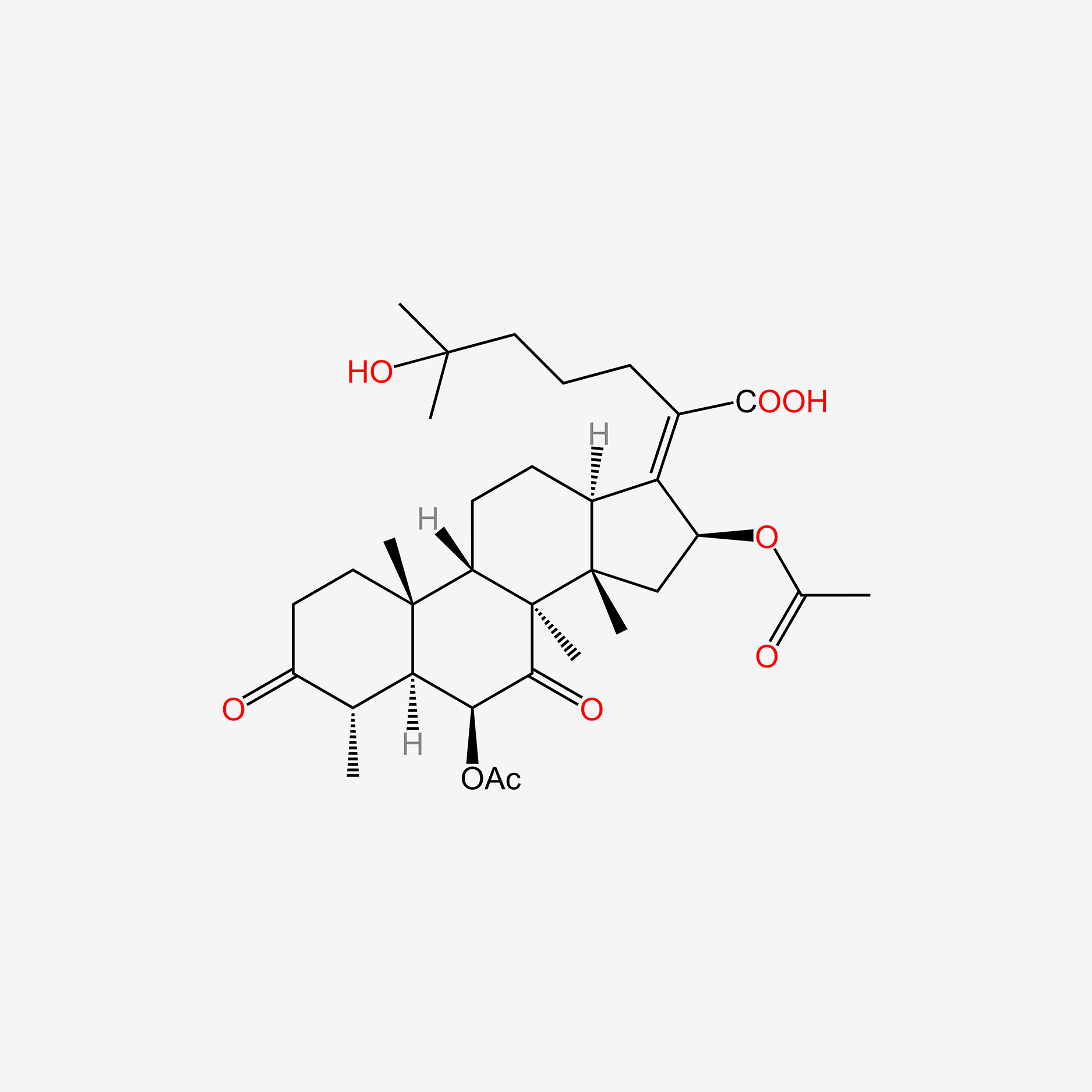 |
0.630 | D0OL7F | 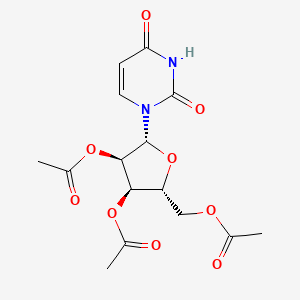 |
0.252 | ||