NPs Basic Information
|
Name |
Sclareol
|
| Molecular Formula | C20H36O2 | |
| IUPAC Name* |
(1R,2R,4aS,8aS)-1-[(3R)-3-hydroxy-3-methylpent-4-enyl]-2,5,5,8a-tetramethyl-3,4,4a,6,7,8-hexahydro-1H-naphthalen-2-ol
|
|
| SMILES |
C[C@]12CCCC([C@@H]1CC[C@@]([C@@H]2CC[C@](C)(C=C)O)(C)O)(C)C
|
|
| InChI |
InChI=1S/C20H36O2/c1-7-18(4,21)13-9-16-19(5)12-8-11-17(2,3)15(19)10-14-20(16,6)22/h7,15-16,21-22H,1,8-14H2,2-6H3/t15-,16+,18-,19-,20+/m0/s1
|
|
| InChIKey |
XVULBTBTFGYVRC-HHUCQEJWSA-N
|
|
| Synonyms |
Sclareol; 515-03-7; SCAREOL; labd-14-ene-8,13-diol; (13R)-Labd-14-ene-8,13-diol; (1R,2R,4aS,8aS)-1-((R)-3-hydroxy-3-methylpent-4-en-1-yl)-2,5,5,8a-tetramethyldecahydronaphthalen-2-ol; B607NP0Q8Y; CHEBI:9053; (1R,2R,4aS,8aS)-1-[(3R)-3-hydroxy-3-methylpent-4-en-1-yl]-2,5,5,8a-tetramethyl-decahydronaphthalen-2-ol; (1R,2R,4aS,8aS)-1-[(3R)-3-hydroxy-3-methylpent-4-enyl]-2,5,5,8a-tetramethyl-3,4,4a,6,7,8-hexahydro-1H-naphthalen-2-ol; Labd-14-ene-8,13-diol, (13R)-; (1R,2R,4aS,8aS)-1-[(3R)-3-hydroxy-3-methylpent-4-en-1-yl]-2,5,5,8a-tetramethyldecahydronaphthalen-2-ol labd-14-ene-8,13-diol; Sclareol (natural); UNII-B607NP0Q8Y; EINECS 208-194-0; Sclareol, 98%; BRN 2054148; SCLAREOL [INCI]; (-)-SCLAREOL; DSSTox_CID_27111; DSSTox_RID_82121; DSSTox_GSID_47111; Sclareol, analytical standard; 4-06-00-05554 (Beilstein Handbook Reference); SCHEMBL873931; CHEMBL294740; DTXSID0047111; FEMA NO. 4502; 1-NAPHTHALENEPROPANOL, .ALPHA.-ETHENYLDECAHYDRO-2-HYDROXY-.ALPHA.,2,5,5,8A-PENTAMETHYL-, (1R-(1.ALPHA.(R*),2.BETA.,4A.BETA.,8A.ALPHA.))-; HY-N0128; ZINC3881344; Tox21_302727; MFCD00869558; Sclareol 1000 microg/mL in Acetone; AKOS025310185; LMPR0104030010; (13R)-Labd-14-ene-8alpha,13-diol; (1R,2R,8aS)-Decahydro-1-(3-hydroxy-3-methyl-4-pentenyl)-2,5,5,8a-tetramethyl-2-naphthol; NCGC00256908-01; 1-Naphthalenepropanol, .alpha.-ethenyldecahydro-2-hydroxy-.alpha.,2,5,5,8a-pentamethyl-, (.alpha.R,1R,2R,4aS,8aS)-; AC-34890; AS-14857; CAS-515-03-7; CS-0007834; S0916; C09183; 515S037; A828631; Q63396017; (1R,2R,4As,8aR)-1-[(3R)-3-hydroxy-3-methylpent-4-enyl]-2,5,5,8a-tetramethyl-3,4,4a,6,7,8-hexahydro-1H-naphthalen-2-ol; (1R,2R,4aS,8aS)-1-[(3R)-3-hydroxy-3-methyl-pent-4-enyl]-2,5,5,8a-tetramethyl-decalin-2-ol; (1R,2R,4aS,8aS)-1-[(3R)-3-hydroxy-3-methylpent-4-en-1-yl]-2,5,5,8a-tetramethyldecahydronaphthalen-2-ol; (1R,2R,8aS)-1-((R)-3-hydroxy-3-methylpent-4-enyl)-2,5,5,8a-tetramethyldecahydronaphthalen-2-ol; (1R-(1alpha(R*),2beta,4Abeta,8aalpha))-2-hydroxy-alpha,2,5,5,8a-pentamethyl-alpha-vinyldecahydronaphthalene-1-propan-1-ol; 1-Naphthalenepropanol, alpha-ethenyldecahydro-2-hydroxy-alpha,2,5,5,8a-pentamethyl-, (1theta-(1alpha(theta),2beta,4abeta,8aalpha))-; 1-Naphthalenepropanol, alpha-ethenyldecahydro-2-hydroxy-alpha,2,5,5,8a-pentamethyl-, (alphaR,1R,2R,4aS,8aS)-; 1-Naphthalenepropanol, alpha-ethenyldecahydro-2-hydroxy-alpha,2,5,5,8a-pentamethyl-, (alphaR,1R,2R,4aS,8aS)-: (1R,2R,4aS,8aS)-1-[(3R)-3-hydroxy-3-methylpent-4-en-1-yl]-2,5,5,8a-tetramethyldecahydronaphthalen-2-ol; 1-Naphthalenepropanol, decahydro-alpha-ethenyl-2-hydroxy-alpha,2,5,5,8a-pentamethyl-, (1R-(1-alpha(R*),2-beta,4a-beta,8a-alpha))-; 86023-49-6
|
|
| CAS | 515-03-7 | |
| PubChem CID | 163263 | |
| ChEMBL ID | CHEMBL294740 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 308.5 | ALogp: | 4.9 |
| HBD: | 2 | HBA: | 2 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 40.5 | Aromatic Rings: | 2 |
| Heavy Atoms: | 22 | QED Weighted: | 0.706 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.53 | MDCK Permeability: | 0.00001620 |
| Pgp-inhibitor: | 0.759 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.008 | 20% Bioavailability (F20%): | 0.089 |
| 30% Bioavailability (F30%): | 0.021 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.219 | Plasma Protein Binding (PPB): | 91.31% |
| Volume Distribution (VD): | 1.233 | Fu: | 9.87% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.03 | CYP1A2-substrate: | 0.314 |
| CYP2C19-inhibitor: | 0.115 | CYP2C19-substrate: | 0.899 |
| CYP2C9-inhibitor: | 0.193 | CYP2C9-substrate: | 0.598 |
| CYP2D6-inhibitor: | 0.047 | CYP2D6-substrate: | 0.296 |
| CYP3A4-inhibitor: | 0.727 | CYP3A4-substrate: | 0.386 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 4.886 | Half-life (T1/2): | 0.241 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.024 | Human Hepatotoxicity (H-HT): | 0.032 |
| Drug-inuced Liver Injury (DILI): | 0.019 | AMES Toxicity: | 0.003 |
| Rat Oral Acute Toxicity: | 0.074 | Maximum Recommended Daily Dose: | 0.043 |
| Skin Sensitization: | 0.709 | Carcinogencity: | 0.062 |
| Eye Corrosion: | 0.975 | Eye Irritation: | 0.915 |
| Respiratory Toxicity: | 0.709 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
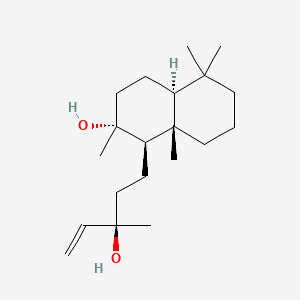
| ENC003102 | 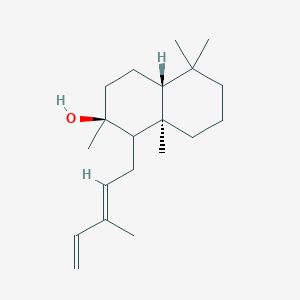 |
0.629 | D07QKN | 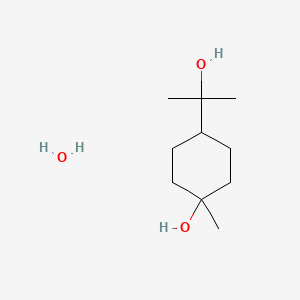 |
0.268 | ||
| ENC002923 | 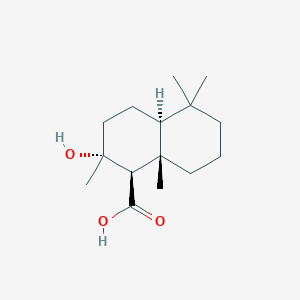 |
0.544 | D02VPX |  |
0.255 | ||
| ENC002918 |  |
0.514 | D05BTM |  |
0.250 | ||
| ENC002266 |  |
0.474 | D0T2PL | 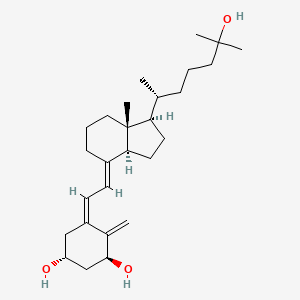 |
0.250 | ||
| ENC002322 | 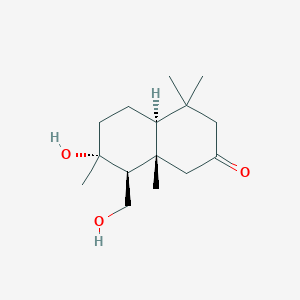 |
0.438 | D0Q6NZ | 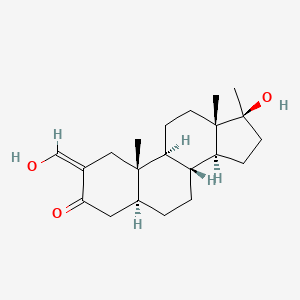 |
0.240 | ||
| ENC002608 | 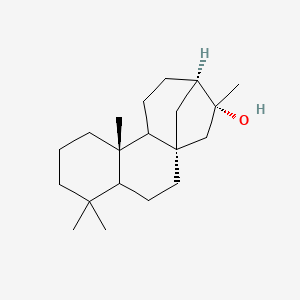 |
0.432 | D02ZGI | 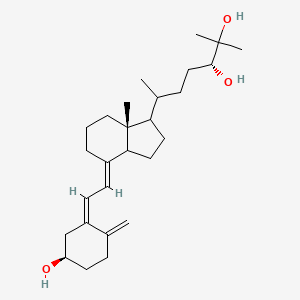 |
0.239 | ||
| ENC001070 | 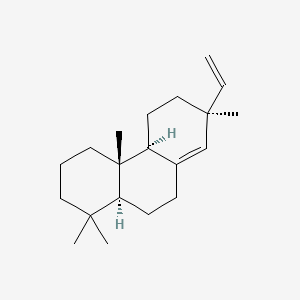 |
0.430 | D0U3GL | 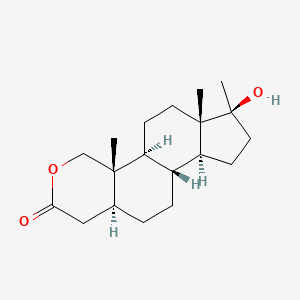 |
0.227 | ||
| ENC001452 | 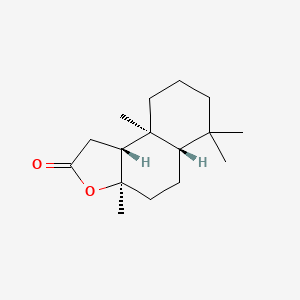 |
0.427 | D0Z1XD | 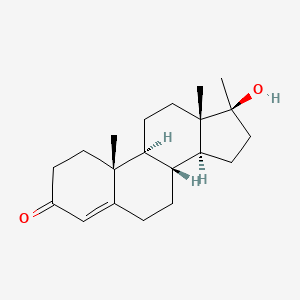 |
0.227 | ||
| ENC000956 | 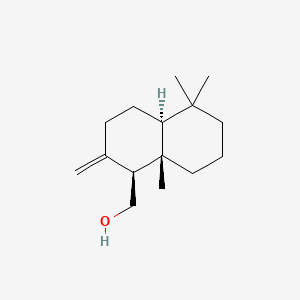 |
0.423 | D0H2MO | 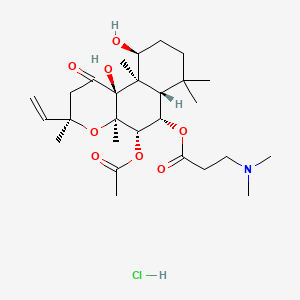 |
0.224 | ||
| ENC002141 | 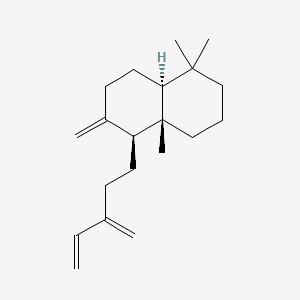 |
0.418 | D04VIS |  |
0.223 | ||