NPs Basic Information
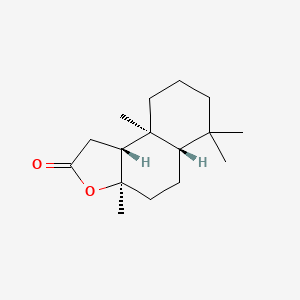
|
Name |
Sclareolide
|
| Molecular Formula | C16H26O2 | |
| IUPAC Name* |
(3aR,5aS,9aS,9bR)-3a,6,6,9a-tetramethyl-1,4,5,5a,7,8,9,9b-octahydrobenzo[e][1]benzofuran-2-one
|
|
| SMILES |
C[C@]12CCCC([C@@H]1CC[C@@]3([C@@H]2CC(=O)O3)C)(C)C
|
|
| InChI |
InChI=1S/C16H26O2/c1-14(2)7-5-8-15(3)11(14)6-9-16(4)12(15)10-13(17)18-16/h11-12H,5-10H2,1-4H3/t11-,12+,15-,16+/m0/s1
|
|
| InChIKey |
IMKJGXCIJJXALX-SHUKQUCYSA-N
|
|
| Synonyms |
Sclareolide; 564-20-5; (3aR)-(+)-Sclareolide; Norambreinolide; 12-Norambreinolide; CLAREOLIDE; (+)-sclareolide; (3aR,5aS,9aS,9bR)-3a,6,6,9a-tetramethyldecahydronaphtho[2,1-b]furan-2(1H)-one; MLS001173371; (3aR,5aS,9aS,9bR)-3a,6,6,9a-tetramethyl-1,4,5,5a,7,8,9,9b-octahydrobenzo[e][1]benzofuran-2-one; 37W4O0O6E6; SMR000538921; (3aR,5aS,9aS,9bR)-Decahydro-3a,6,6,9a-tetramethylnaphtho[2,1-b]furan-2(1H)-one; Decahydrotetramethylnaphthofuranone; Norambreinolide, (+)-; UNII-37W4O0O6E6; Naphtho(2,1-b)furan-2(1H)-one, decahydro-3a,6,6,9a-tetramethyl-, (3aR,5aS,9aS,9bR)-; EINECS 209-269-0; CLARY SAGE LACTONE; SCLAREOLIDE [FHFI]; SCLAREOLIDE [INCI]; DSSTox_CID_27686; DSSTox_RID_82502; (3a,R)-(+)-Sclareolide; DSSTox_GSID_47686; SCHEMBL83395; Sclareolide (Norambreinolide); Sclareolide - Norambreinolide; CHEMBL304461; cid_929262; DTXSID8047686; FEMA NO. 3794; BDBM75178; CHEBI:156168; HMS2860M07; ZINC519827; (3aR)-(+)-Sclareolide, 97%; HY-N0129; Tox21_302544; MFCD00134168; s2355; AKOS015901397; CCG-208493; DS-7369; NCGC00163620-01; NCGC00163620-03; NCGC00256856-01; 3a,4,5,5aalpha,6,7,8,9,9a,9balpha-decahydro-3abeta,6,6,9abeta-tetramethylnaphtho(2,1-b)furan-2(1H)-one; CAS-564-20-5; Naphtho(2,1-b)furan-2(1H)-one, 3a,4,5,5aalpha,6,7,8,9,9a,9balpha-decahydro-3abeta,6,6,9abeta-tetramethyl-; Naphtho(2,1-b)furan-2(1H)-one, decahydro-3a,6,6,9a-tetramethyl-, (3aR-(3aalpha,5abeta,9aalpha,9bbeta))-; (3aR)-(+)-Sclareolide, analytical standard; CS-0007855; S0847; (3aR)-(+)-Sclareolide, natural, 97%, FG; H10516; 564S205; A869953; SR-01000812893; SR-01000812893-3; BRD-K72925150-001-11-1; (3aR,5aS,9aS,9bR)-3a,6,6,9a-tetramethyl-1,4,5,5a,7,8,9,9b-octahydrobenzo[e][1]benzouran-2-one; (3aR,5aS,9aS,9bR)-3a,6,6,9a-tetramethyl-1,4,5,5a,7,8,9,9b-octahydrobenzo[e]benzofuran-2-one; (3AR-(3aalpha,5abeta,9aalpha,9bbeta))decahydro-3a,6,6,9a-tetramethylnaphth(2,1-b)furan-2(1H)-one
|
|
| CAS | 564-20-5 | |
| PubChem CID | 929262 | |
| ChEMBL ID | CHEMBL304461 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 250.38 | ALogp: | 4.3 |
| HBD: | 0 | HBA: | 2 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 26.3 | Aromatic Rings: | 3 |
| Heavy Atoms: | 18 | QED Weighted: | 0.588 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.743 | MDCK Permeability: | 0.00002240 |
| Pgp-inhibitor: | 0.954 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.585 |
| 30% Bioavailability (F30%): | 0.953 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.15 | Plasma Protein Binding (PPB): | 87.08% |
| Volume Distribution (VD): | 0.573 | Fu: | 25.62% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.078 | CYP1A2-substrate: | 0.305 |
| CYP2C19-inhibitor: | 0.185 | CYP2C19-substrate: | 0.879 |
| CYP2C9-inhibitor: | 0.245 | CYP2C9-substrate: | 0.154 |
| CYP2D6-inhibitor: | 0.015 | CYP2D6-substrate: | 0.575 |
| CYP3A4-inhibitor: | 0.261 | CYP3A4-substrate: | 0.333 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 9.519 | Half-life (T1/2): | 0.313 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.049 | Human Hepatotoxicity (H-HT): | 0.136 |
| Drug-inuced Liver Injury (DILI): | 0.134 | AMES Toxicity: | 0.006 |
| Rat Oral Acute Toxicity: | 0.477 | Maximum Recommended Daily Dose: | 0.21 |
| Skin Sensitization: | 0.875 | Carcinogencity: | 0.249 |
| Eye Corrosion: | 0.926 | Eye Irritation: | 0.646 |
| Respiratory Toxicity: | 0.962 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC002923 | 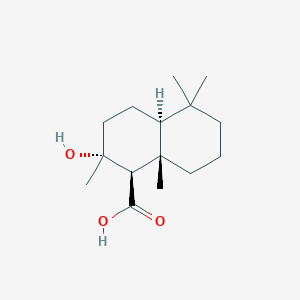 |
0.524 | D0U3GL | 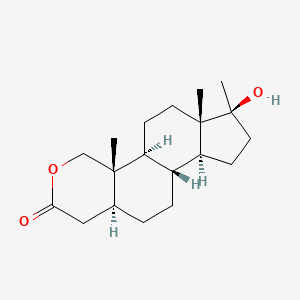 |
0.325 | ||
| ENC003162 | 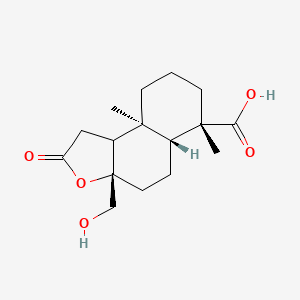 |
0.514 | D0H1QY |  |
0.300 | ||
| ENC002266 |  |
0.493 | D0C7JF |  |
0.291 | ||
| ENC002608 |  |
0.486 | D0Z1XD |  |
0.279 | ||
| ENC003145 | 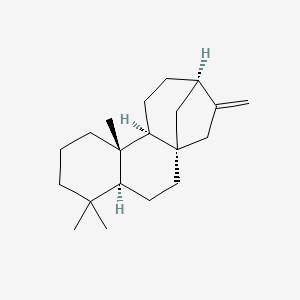 |
0.479 | D0Q6NZ | 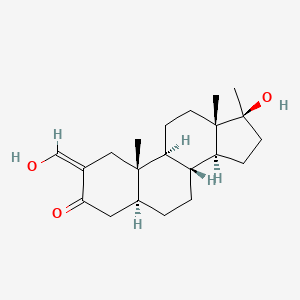 |
0.278 | ||
| ENC001070 | 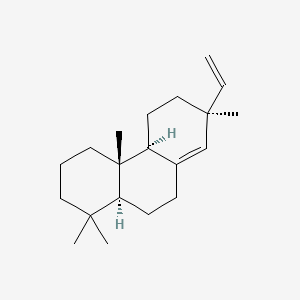 |
0.465 | D0EP0C | 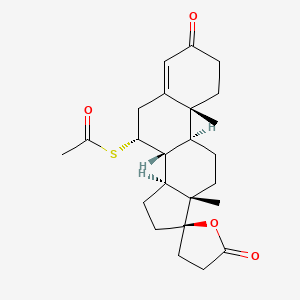 |
0.262 | ||
| ENC001193 | 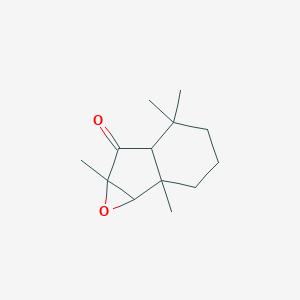 |
0.459 | D04GJN | 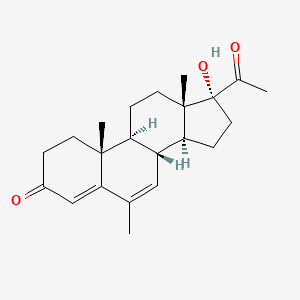 |
0.258 | ||
| ENC003102 | 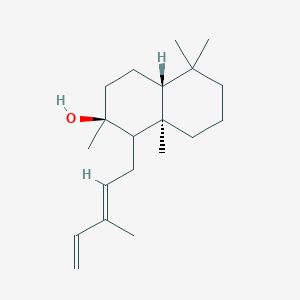 |
0.438 | D0I2SD | 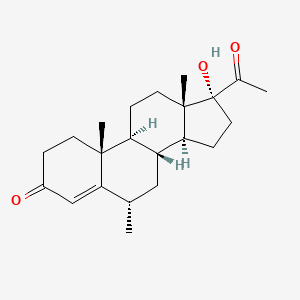 |
0.258 | ||
| ENC000956 | 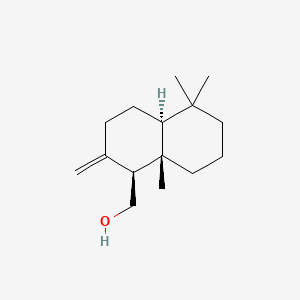 |
0.438 | D04DJN | 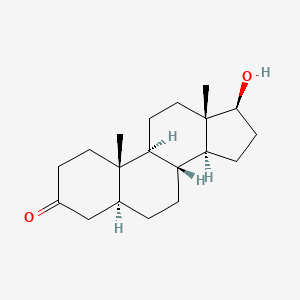 |
0.256 | ||
| ENC001075 | 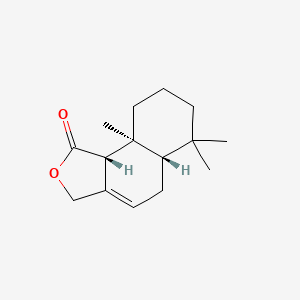 |
0.433 | D0Q4SD | 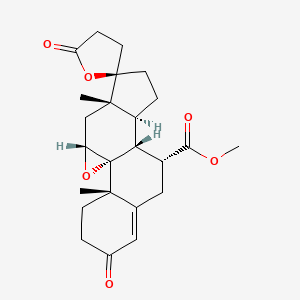 |
0.255 | ||