NPs Basic Information
|
Name |
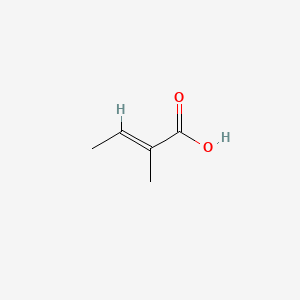
Tiglic acid
|
| Molecular Formula | C5H8O2 | |
| IUPAC Name* |
(E)-2-methylbut-2-enoic acid
|
|
| SMILES |
C/C=C(\C)/C(=O)O
|
|
| InChI |
InChI=1S/C5H8O2/c1-3-4(2)5(6)7/h3H,1-2H3,(H,6,7)/b4-3+
|
|
| InChIKey |
UIERETOOQGIECD-ONEGZZNKSA-N
|
|
| Synonyms |
TIGLIC ACID; 80-59-1; Tiglinic acid; Cevadic acid; 2-methylbut-2-enoic acid; trans-2,3-Dimethylacrylic acid; (E)-2-Methylbut-2-enoic acid; 2-Methyl-2-butenoic acid; trans-2-Methylcrotonic acid; trans-2-Methyl-2-butenoic acid; (E)-2,3-Dimethylacrylic acid; (E)-2-Methylcrotonic acid; (E)-2-Methyl-2-butenoic acid; 2-Butenoic acid, 2-methyl-, (2E)-; Crotonic acid, 2-methyl-, (E)-; tiglate; alpha-Methylcrotonic acid; 2-Butenoic acid, 2-methyl-, (E)-; (2E)-2-methylbut-2-enoic acid; 2,3-Dimethylacrylic acid, (E)-; 2-Methylcrotonic acid; trans-alpha,beta-Dimethylacrylic acid; FEMA No. 3599; 13201-46-2; NSC 44235; (E)-2-methyl-Crotonic acid; 2,3-Dimethylacrylic acid; 2-methyl-2E-butenoic acid; Tiglicacid; trans-.alpha.,.beta.-Dimethylacrylic acid; CHEBI:9592; 2-methyl-(E)-2-butenoic acid; alpha,beta-dimethyl acrylic acid; NSC8999; I5792N03HC; NSC-8999; NSC44235; NSC-44235; sabadillic acid; methyl methacrylic acid; alpha-Methylcrotonic acid, (E)-; 2-Methyl-2-butenoic acid, (E)-; EINECS 201-295-0; MFCD00066864; BRN 1236500; Tiglinsaeure; Cevadate; Tiglinate; epsilon-Tiglate; UNII-I5792N03HC; AI3-36118; E-Tiglate; Methylbutenoicacid; E-Tiglic acid; HSDB 7614; (2E)-2-Methyl-2-butenoic acid; (E)-tiglic acid; 2-methyl-Crotonate; epsilon-Tiglic acid; EINECS 236-167-3; methyl crotonic acid; methylmethacrylic acid; 2,3-Dimethylacrylate; 2-methyl-2-butenoate; Tiglic acid, (E)-; 2-Methylbut-2-enoate; 2-methyl-Crotonic acid; trans-2-Methylcrotonate; (E)-2-Methylcrotonate; 2-methylbut-2-enoicacid; (E)-2-methyl-Crotonate; alpha-methyl-crotonic acid; TIGLIC ACID [MI]; bmse000727; TIGLIC ACID [HSDB]; trans-2,3-Dimethylacrylate; (E)-2,3-Dimethylacrylate; 2-Butenoic acid,2-methyl-; 2-methyl-(E)-2-butenoate; trans-2-Methyl-2-butenoate; (E)-2-methyl-2-Butenoate; SCHEMBL15042; 4-02-00-01552 (Beilstein Handbook Reference); CHEMBL52416; (2E)-2-Methyl-2-butenoate; GTPL6499; trans-Crotonic acid, 2-methyl-; CHEBI:36432; trans-alpha,beta-Dimethylacrylate; DTXSID80883257; E)-2-METHYLCROTONIC ACID; trans-2-methyl-but-2-enoic acid; ZINC897443; BCP18945; (2E)-2-Methyl-2-butenoic acid #; LMFA01020030; s3789; AKOS003375681; CCG-266028; CS-W013715; HY-W012999; trans-2,3-Dimethylacrylic acid, 98%; (E)-.ALPHA.-METHYLCROTONIC ACID; LS-13047; 2-METHYLBUT-2-ENOIC ACID, (E)-; METHYL-2-BUTENOIC ACID, TRANS-2-; CS-0356606; T0246; EN300-83150; C08279; EN300-370249; TRANS-2-METHYL-2-BUTENOIC ACID [FHFI]; trans-2-Methyl-2-butenoic acid, >=99%, FG; A839954; Q425475; Q27116830; F0001-2086; trans-2-Methylcrotonic acid = trans-2-Methyl-2-butenoate; trans-2-Methylcrotonic acid = trans-2-Methyl-2-butenoic acid; trans-2-Methylcrotonic acid = trans-2-Methyl-2-butenoic acid = Tiglinate; alpha,beta-dimethyl acrylic acid; 2-Methyl-2-butenoic acid; (E)-2-methyl-Crotonic acid; trans-2-Methylcrotonic acid = trans-2-Methyl-2-butenoic acid = Tiglinic acid
|
|
| CAS | 80-59-1 | |
| PubChem CID | 125468 | |
| ChEMBL ID | CHEMBL52416 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 100.12 | ALogp: | 1.0 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 37.3 | Aromatic Rings: | 0 |
| Heavy Atoms: | 7 | QED Weighted: | 0.507 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.588 | MDCK Permeability: | 0.00002670 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.003 |
| Human Intestinal Absorption (HIA): | 0.009 | 20% Bioavailability (F20%): | 0.003 |
| 30% Bioavailability (F30%): | 0.04 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.544 | Plasma Protein Binding (PPB): | 33.31% |
| Volume Distribution (VD): | 0.317 | Fu: | 67.87% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.046 | CYP1A2-substrate: | 0.471 |
| CYP2C19-inhibitor: | 0.051 | CYP2C19-substrate: | 0.072 |
| CYP2C9-inhibitor: | 0.054 | CYP2C9-substrate: | 0.317 |
| CYP2D6-inhibitor: | 0.028 | CYP2D6-substrate: | 0.187 |
| CYP3A4-inhibitor: | 0.012 | CYP3A4-substrate: | 0.074 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 5.154 | Half-life (T1/2): | 0.897 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.013 | Human Hepatotoxicity (H-HT): | 0.271 |
| Drug-inuced Liver Injury (DILI): | 0.185 | AMES Toxicity: | 0.01 |
| Rat Oral Acute Toxicity: | 0.41 | Maximum Recommended Daily Dose: | 0.015 |
| Skin Sensitization: | 0.503 | Carcinogencity: | 0.126 |
| Eye Corrosion: | 0.968 | Eye Irritation: | 0.996 |
| Respiratory Toxicity: | 0.046 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001585 | 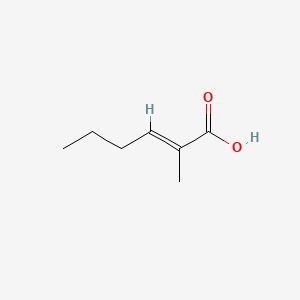 |
0.481 | D0G4JI | 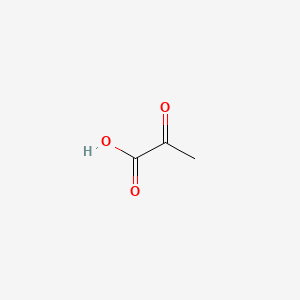 |
0.429 | ||
| ENC000061 | 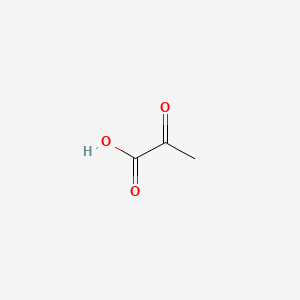 |
0.429 | D04CRL | 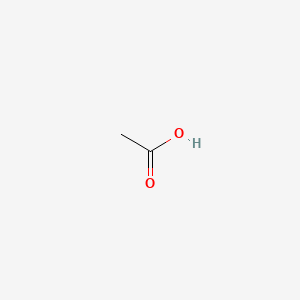 |
0.389 | ||
| ENC001556 | 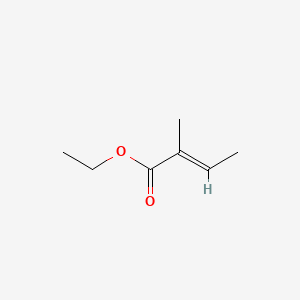 |
0.429 | D0R9BG | 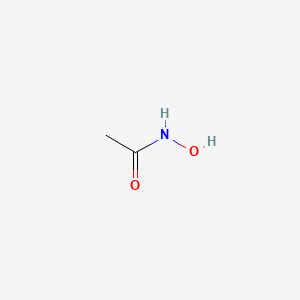 |
0.273 | ||
| ENC000009 | 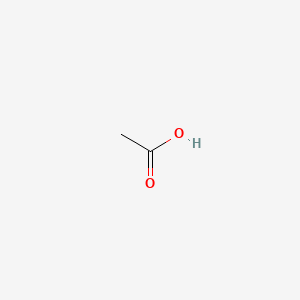 |
0.389 | D0Z4NI | 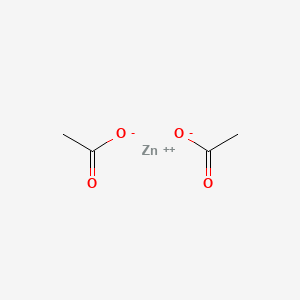 |
0.259 | ||
| ENC001629 | 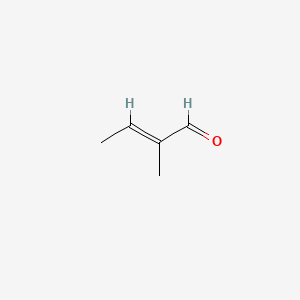 |
0.333 | D0F1GS | 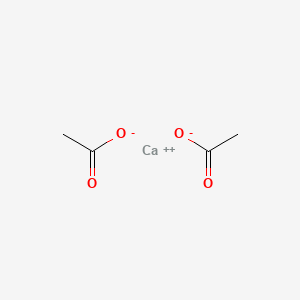 |
0.259 | ||
| ENC001727 | 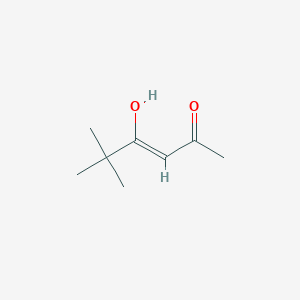 |
0.323 | D09PUL | 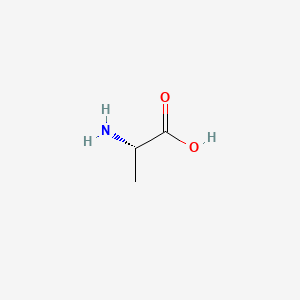 |
0.250 | ||
| ENC000313 | 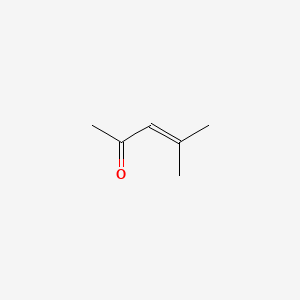 |
0.308 | D08QGD | 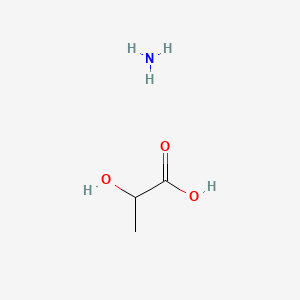 |
0.240 | ||
| ENC000149 | 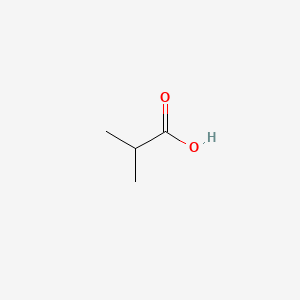 |
0.304 | D0Z4UY | 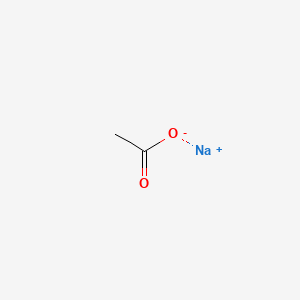 |
0.238 | ||
| ENC000010 | 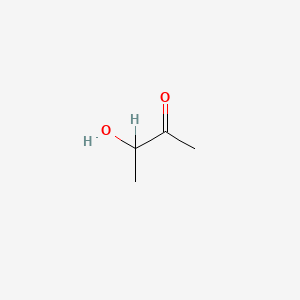 |
0.304 | D0C1PY | 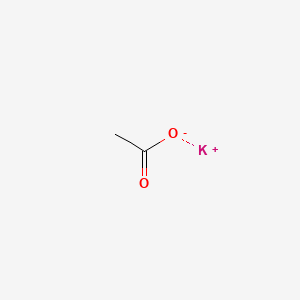 |
0.238 | ||
| ENC000001 | 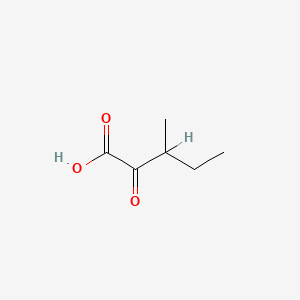 |
0.300 | D02FLB |  |
0.238 | ||