NPs Basic Information
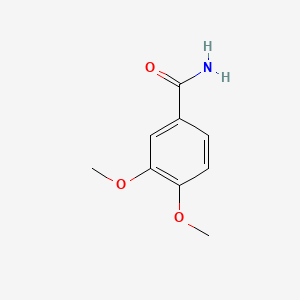
|
Name |
3,4-Dimethoxybenzamide
|
| Molecular Formula | C9H11NO3 | |
| IUPAC Name* |
3,4-dimethoxybenzamide
|
|
| SMILES |
COC1=C(C=C(C=C1)C(=O)N)OC
|
|
| InChI |
InChI=1S/C9H11NO3/c1-12-7-4-3-6(9(10)11)5-8(7)13-2/h3-5H,1-2H3,(H2,10,11)
|
|
| InChIKey |
XNDZRGTVUVVHQT-UHFFFAOYSA-N
|
|
| Synonyms |
3,4-Dimethoxybenzamide; 1521-41-1; Veratramide; Benzamide, 3,4-dimethoxy-; Veratrimidic acid; 3,4-Dimethoxy-benzamide; SEW9F1FKI8; MFCD00017128; NSC-370837; Veratramide; Veratrimidic acid; 3,4-Dimethoxybenzamide; NSC 370837; EINECS 216-190-5; NSC 370837; BRN 2646714; NSC370837; UNII-SEW9F1FKI8; Oprea1_176663; Oprea1_802187; 3-10-00-01427 (Beilstein Handbook Reference); SCHEMBL335681; CHEMBL505584; DTXSID1074549; ZINC298681; AMY28152; HY-N1777; AKOS000593686; SR-4191; AS-57779; SY275554; CS-0017631; FT-0656647; A809265; J-511211; Z33546772
|
|
| CAS | 1521-41-1 | |
| PubChem CID | 73705 | |
| ChEMBL ID | CHEMBL505584 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 181.19 | ALogp: | 1.0 |
| HBD: | 1 | HBA: | 3 |
| Rotatable Bonds: | 3 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 61.6 | Aromatic Rings: | 1 |
| Heavy Atoms: | 13 | QED Weighted: | 0.762 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.714 | MDCK Permeability: | 0.00002290 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.405 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.003 |
| 30% Bioavailability (F30%): | 0.353 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.994 | Plasma Protein Binding (PPB): | 60.13% |
| Volume Distribution (VD): | 1.034 | Fu: | 44.81% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.678 | CYP1A2-substrate: | 0.907 |
| CYP2C19-inhibitor: | 0.062 | CYP2C19-substrate: | 0.4 |
| CYP2C9-inhibitor: | 0.021 | CYP2C9-substrate: | 0.665 |
| CYP2D6-inhibitor: | 0.019 | CYP2D6-substrate: | 0.729 |
| CYP3A4-inhibitor: | 0.019 | CYP3A4-substrate: | 0.252 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.909 | Half-life (T1/2): | 0.638 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.142 | Human Hepatotoxicity (H-HT): | 0.155 |
| Drug-inuced Liver Injury (DILI): | 0.547 | AMES Toxicity: | 0.017 |
| Rat Oral Acute Toxicity: | 0.025 | Maximum Recommended Daily Dose: | 0.019 |
| Skin Sensitization: | 0.084 | Carcinogencity: | 0.276 |
| Eye Corrosion: | 0.006 | Eye Irritation: | 0.824 |
| Respiratory Toxicity: | 0.023 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000478 | 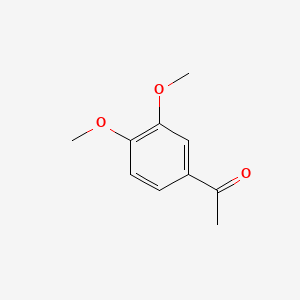 |
0.750 | D02XJY |  |
0.400 | ||
| ENC000499 | 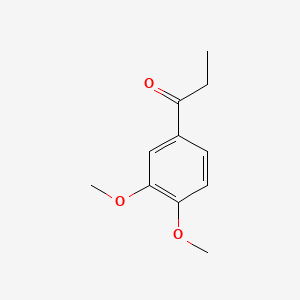 |
0.698 | D09GYT | 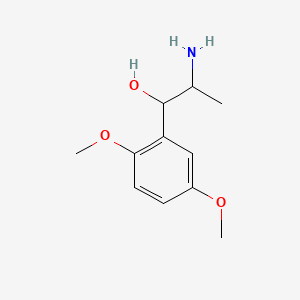 |
0.389 | ||
| ENC001055 | 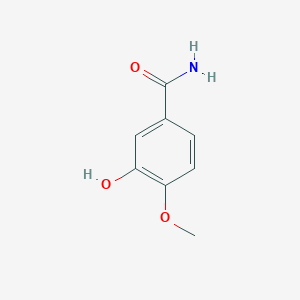 |
0.634 | D0E6OC | 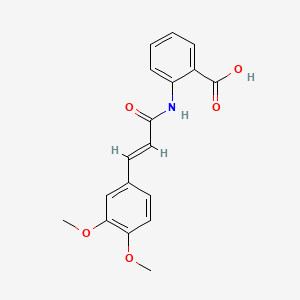 |
0.360 | ||
| ENC001056 | 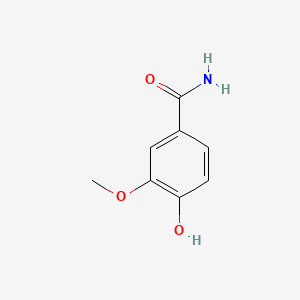 |
0.634 | D0E9CD |  |
0.354 | ||
| ENC000501 | 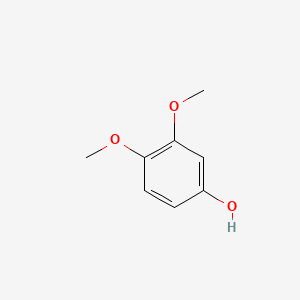 |
0.585 | D0FN7J | 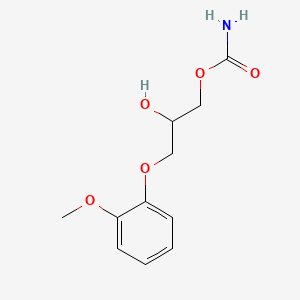 |
0.344 | ||
| ENC001363 | 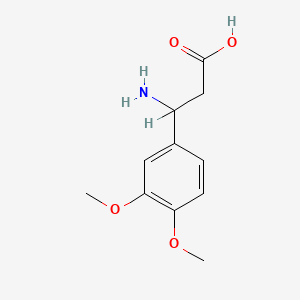 |
0.560 | D0L0ZF | 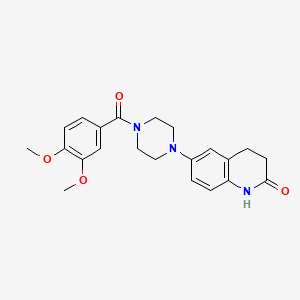 |
0.341 | ||
| ENC001461 | 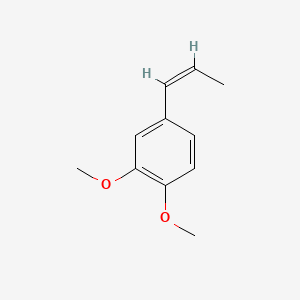 |
0.511 | D0VU8Q | 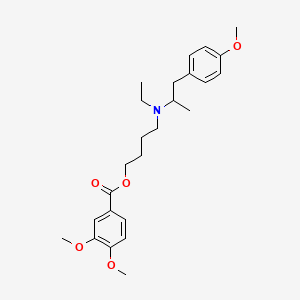 |
0.326 | ||
| ENC004830 | 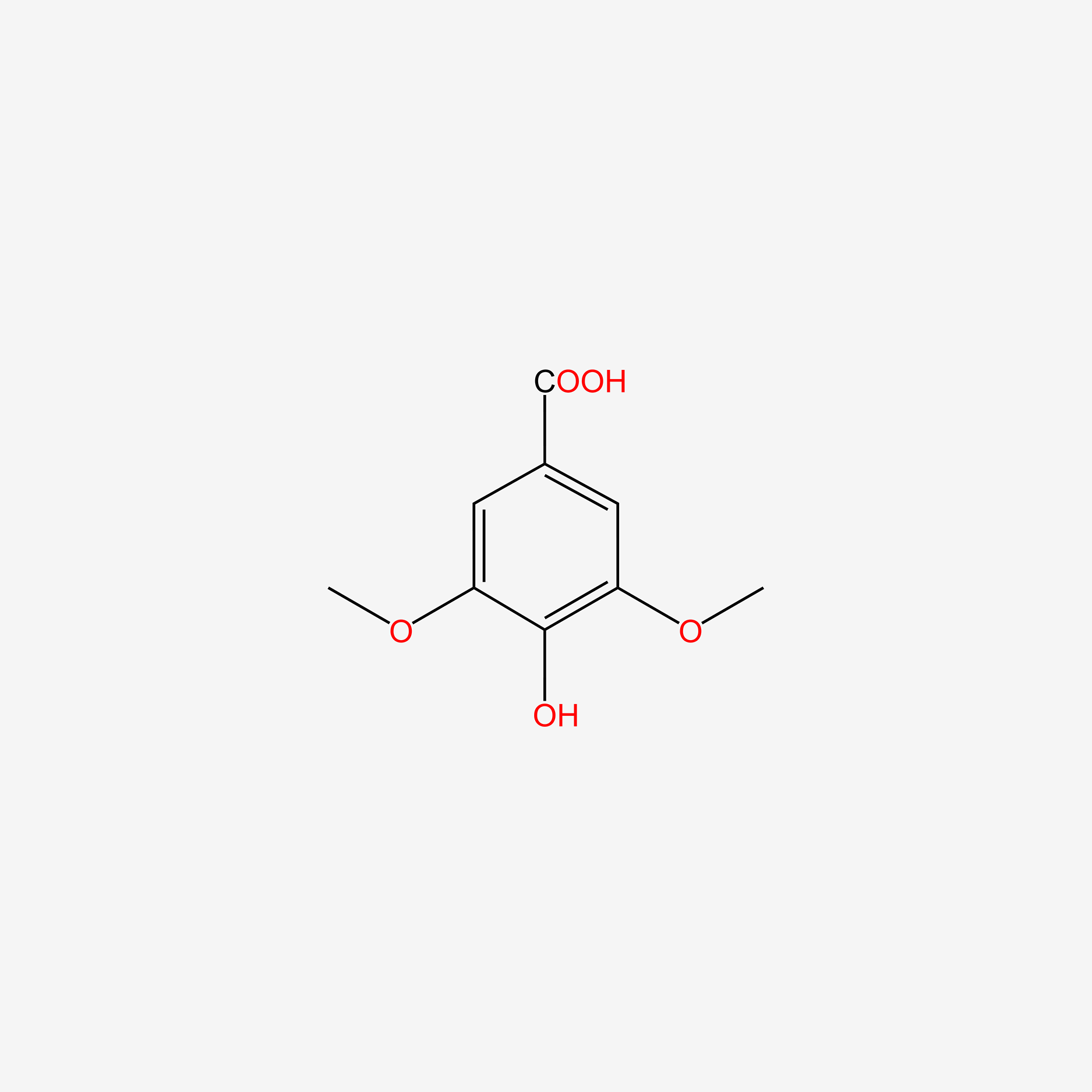 |
0.469 | D06GCK |  |
0.316 | ||
| ENC000367 | 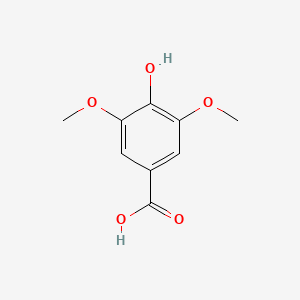 |
0.469 | D0Q9ON |  |
0.316 | ||
| ENC000296 |  |
0.457 | D06FPQ |  |
0.303 | ||