NPs Basic Information
|
Name |
Vinylbital
|
| Molecular Formula | C11H16N2O3 | |
| IUPAC Name* |
5-ethenyl-5-pentan-2-yl-1,3-diazinane-2,4,6-trione
|
|
| SMILES |
CCCC(C)C1(C(=O)NC(=O)NC1=O)C=C
|
|
| InChI |
InChI=1S/C11H16N2O3/c1-4-6-7(3)11(5-2)8(14)12-10(16)13-9(11)15/h5,7H,2,4,6H2,1,3H3,(H2,12,13,14,15,16)
|
|
| InChIKey |
KGKJZEKQJQQOTD-UHFFFAOYSA-N
|
|
| Synonyms |
Vinylbital; Vinylbitone; Butylvinal; Speda; Vinylbitalum; Bykonox; 2430-49-1; Optanox; Vinymal; Butyvinyl; JD-96; (-)-Vinylbital; Vinylbital, (+)-; Vinylbital, (-)-; 5-(1-Methylbutyl)-5-vinylbarbituric acid; KI0U0LRM1V; 3J2D3OVU7O; 3W58ITX06Q; 2,4,6(1H,3H,5H)-Pyrimidinetrione, 5-ethenyl-5-(1-methylbutyl)-; Vinylbital (INN); Butyvinal; VINYLBITAL [INN]; 5-Ethenyl-5-(1-methylbutyl)-2,4,6(1H,3H,5H)-pyrimidinetrione; Barbituric acid, 5-(1-methylbutyl)-5-vinyl-, (-)-; Vinilbital; 2,4,6(1H,3H,5H)-Pyrimidinetrione, 5-ethenyl-5-(1-methylbutyl)-, (+)-; 2,4,6(1H,3H,5H)-Pyrimidinetrione, 5-ethenyl-5-(1-methylbutyl)-, (-)-; 5767-33-9; 5821-16-9; Vinilbital [INN-Spanish]; Vinylbitalum [INN-Latin]; UNII-3W58ITX06Q; Vinylbital [INN:BAN:DCF]; EINECS 219-395-8; 5-ethenyl-5-pentan-2-yl-1,3-diazinane-2,4,6-trione; 5-(1-Methylbutyl)-5-vinylbarbitursaeure; VINYLBITAL [MI]; UNII-KI0U0LRM1V; UNII-3J2D3OVU7O; Barbituric acid, 5-(1-methylbutyl)-5-vinyl-; VINYLBITAL [MART.]; VINYLBITAL [WHO-DD]; SCHEMBL156714; JD96; CHEMBL2105552; DTXSID50862938; CHEBI:134922; DB13770; 5-Vinyl-5-(1-methylbutyl)-barbitursaure; D07321; Q410157; 5-(1-Methylbutyl)-5-vinyl-2,4,6(1H,3H,5H)-pyrimidinetrione; 5-(pentan-2-yl)-5-vinylpyrimidine-2,4,6(1H,3H,5H)-trione; 5-Ethenyl-5-(1-methylbutyl)-2,4,6(1H,3H,5H)-pyrimid inetrione; (+)-5-ETHENYL-5-(1-METHYLBUTYL)-2,4,6(1H,3H,5H)-PYRIMIDINETRIONE; (-)-5-ETHENYL-5-(1-METHYLBUTYL)-2,4,6(1H,3H,5H)-PYRIMIDINETRIONE
|
|
| CAS | 2430-49-1 | |
| PubChem CID | 72135 | |
| ChEMBL ID | CHEMBL2105552 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 224.26 | ALogp: | 2.0 |
| HBD: | 2 | HBA: | 3 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 75.3 | Aromatic Rings: | 1 |
| Heavy Atoms: | 16 | QED Weighted: | 0.559 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.664 | MDCK Permeability: | 0.00007230 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.001 |
| 30% Bioavailability (F30%): | 0.001 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 1 | Plasma Protein Binding (PPB): | 51.91% |
| Volume Distribution (VD): | 0.785 | Fu: | 56.88% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.051 | CYP1A2-substrate: | 0.623 |
| CYP2C19-inhibitor: | 0.114 | CYP2C19-substrate: | 0.934 |
| CYP2C9-inhibitor: | 0.05 | CYP2C9-substrate: | 0.426 |
| CYP2D6-inhibitor: | 0.009 | CYP2D6-substrate: | 0.155 |
| CYP3A4-inhibitor: | 0.015 | CYP3A4-substrate: | 0.257 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 0.524 | Half-life (T1/2): | 0.764 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.007 | Human Hepatotoxicity (H-HT): | 0.255 |
| Drug-inuced Liver Injury (DILI): | 0.071 | AMES Toxicity: | 0.422 |
| Rat Oral Acute Toxicity: | 0.966 | Maximum Recommended Daily Dose: | 0.128 |
| Skin Sensitization: | 0.09 | Carcinogencity: | 0.016 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.015 |
| Respiratory Toxicity: | 0.146 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
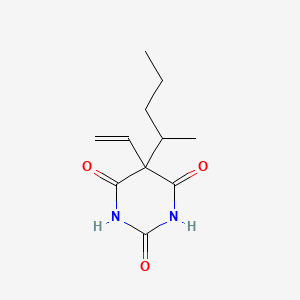
| ENC000121 | 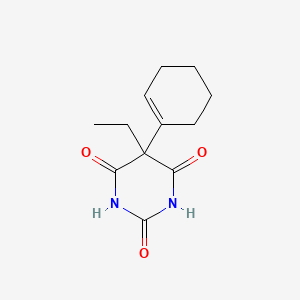 |
0.348 | D00SJE | 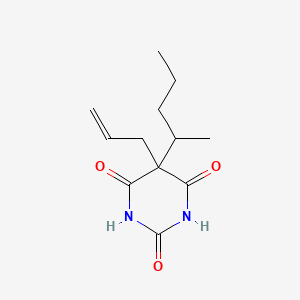 |
0.673 | ||
| ENC004903 | 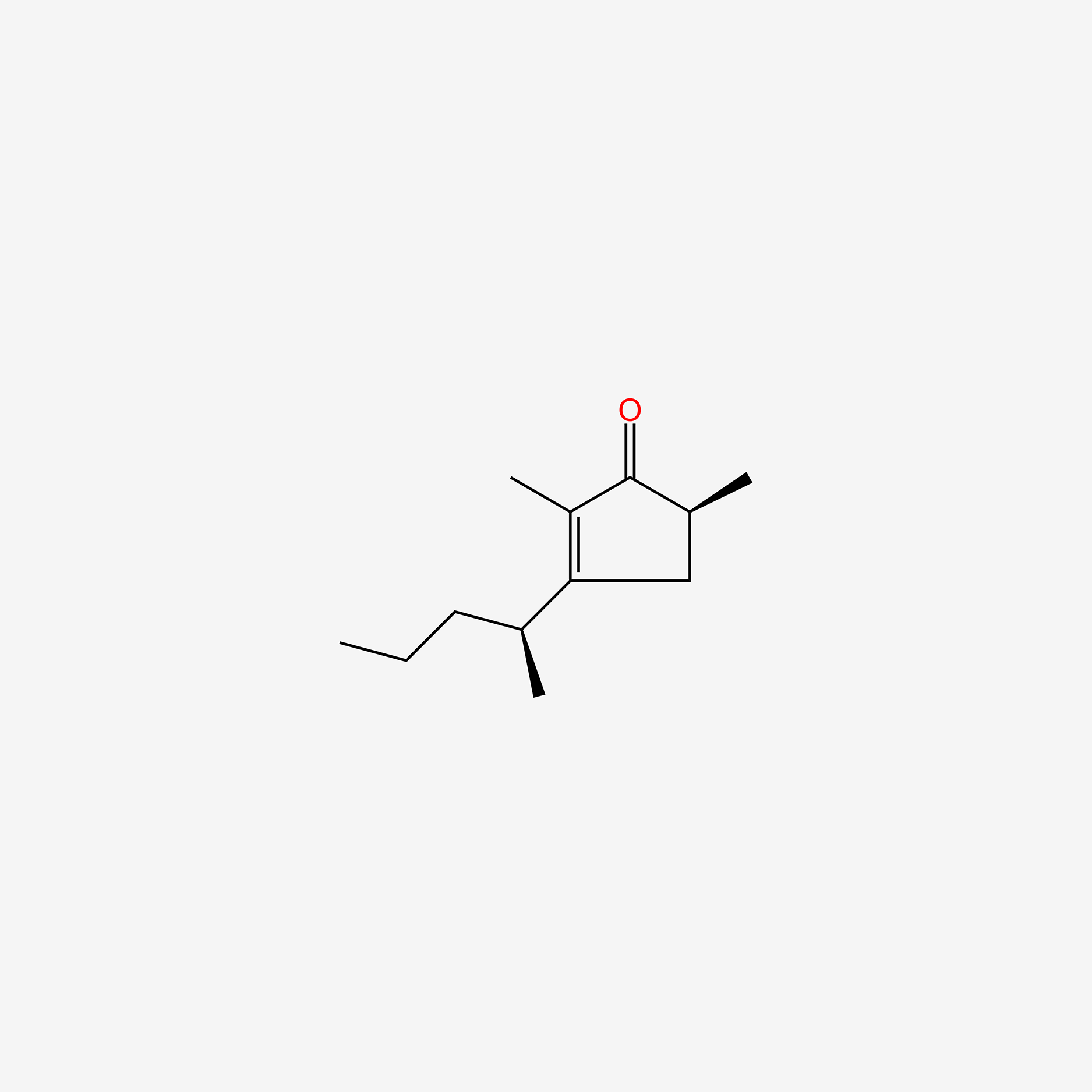 |
0.246 | D0F0YZ | 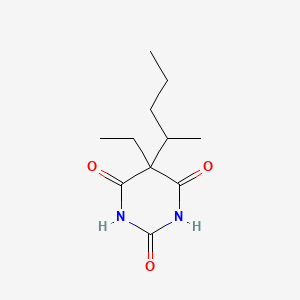 |
0.615 | ||
| ENC002751 | 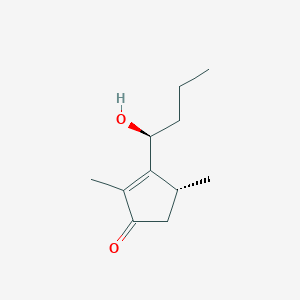 |
0.226 | D0W0MF | 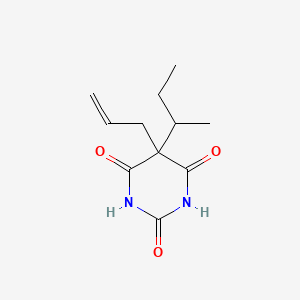 |
0.585 | ||
| ENC001229 | 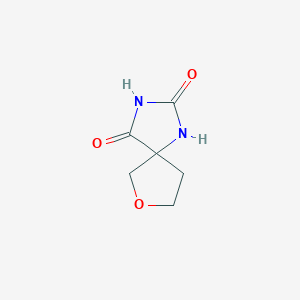 |
0.217 | D0A4JK | 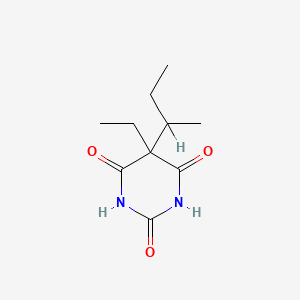 |
0.528 | ||
| ENC004235 |  |
0.211 | D05TMQ | 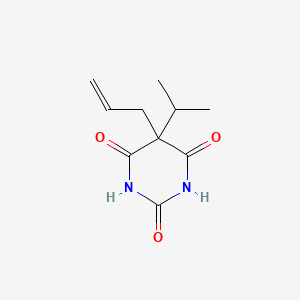 |
0.528 | ||
| ENC002257 | 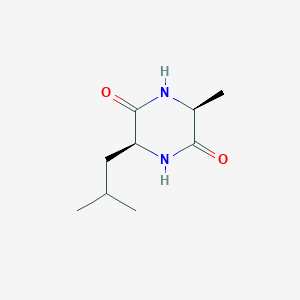 |
0.206 | D05BQK | 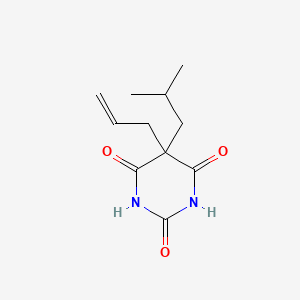 |
0.527 | ||
| ENC004512 | 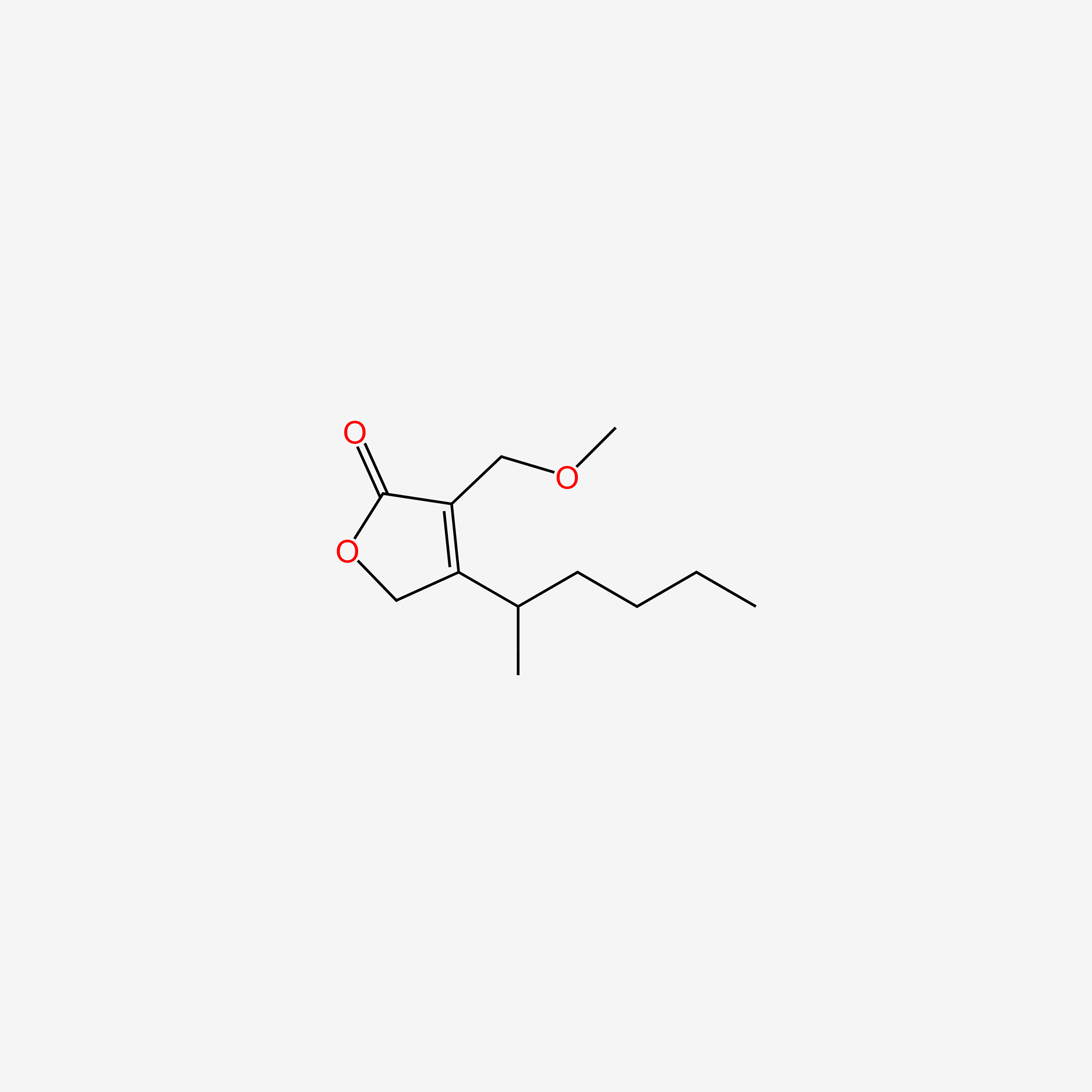 |
0.203 | D0R6BR | 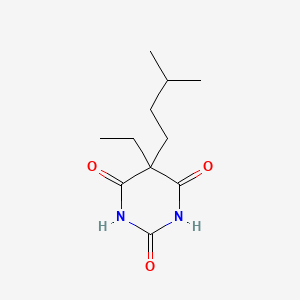 |
0.500 | ||
| ENC002212 | 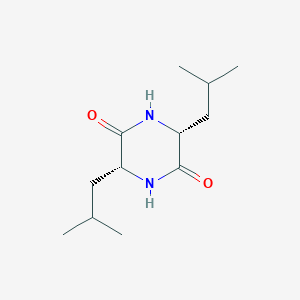 |
0.200 | D06NSA | 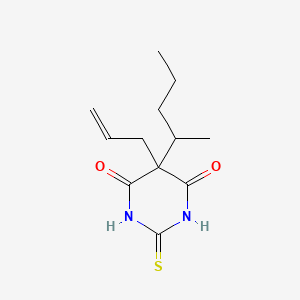 |
0.500 | ||
| ENC002820 | 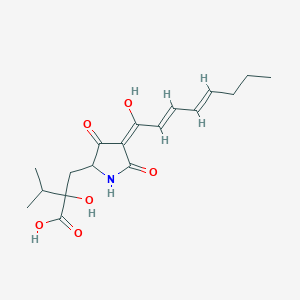 |
0.200 | D0E0WQ | 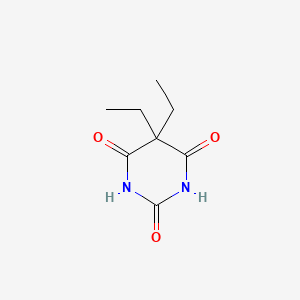 |
0.490 | ||
| ENC000990 | 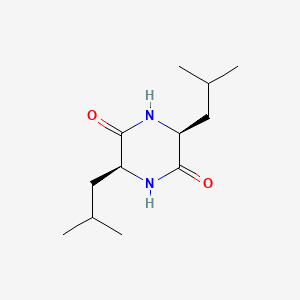 |
0.200 | D0O3AB | 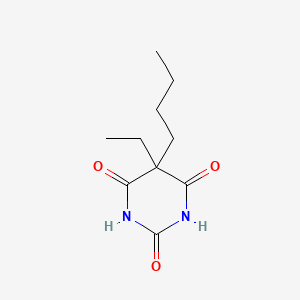 |
0.464 | ||