NPs Basic Information
|
Name |
Carazolol
|
| Molecular Formula | C18H22N2O2 | |
| IUPAC Name* |
1-(9H-carbazol-4-yloxy)-3-(propan-2-ylamino)propan-2-ol
|
|
| SMILES |
CC(C)NCC(COC1=CC=CC2=C1C3=CC=CC=C3N2)O
|
|
| InChI |
InChI=1S/C18H22N2O2/c1-12(2)19-10-13(21)11-22-17-9-5-8-16-18(17)14-6-3-4-7-15(14)20-16/h3-9,12-13,19-21H,10-11H2,1-2H3
|
|
| InChIKey |
BQXQGZPYHWWCEB-UHFFFAOYSA-N
|
|
| Synonyms |
Carazolol; 57775-29-8; Conducton; Suacron; 1-(Carbazol-4-yloxy)-3-(isopropylamino)-2-propanol; BM 51052; corazolol; 1-((9H-Carbazol-4-yl)oxy)-3-(isopropylamino)propan-2-ol; 1-(9H-carbazol-4-yloxy)-3-(propan-2-ylamino)propan-2-ol; BM-51052; Conducton hydrochloride; CHEMBL324665; 2-Propanol, 1-(9H-carbazol-4-yloxy)-3-[(1-methylethyl)amino]-; 29PW75S82A; Carazolol (INN); Carazolol 100 microg/mL in Acetonitrile; CARAZOLOL [INN]; Carazololum; Carazolol [INN:BAN]; 2-Propanol, 1-(9H-carbazol-4-yloxy)-3-((1-methylethyl)amino)-; Carazololum [INN-Latin]; CCRIS 1047; 1-(9H-carbazol-4-yloxy)-3-(isopropylamino)propan-2-ol; EINECS 260-945-1; BRN 3620576; UNII-29PW75S82A; Conducton (TN); 1-(4-Carbazolyloxy)-3-isopropylamino-2-propanol; 4-(2-Hydroxy-3-isopropylamino-propoxy)-carbazole; 1-(9H-Carbazol-4-yloxy-3-((1-methylethyl)amino)-2-propanol); CARAZOLOL [MI]; CARAZOLOL [MART.]; CARAZOLOL [WHO-DD]; (isopropylamino)propan-2-ol; Suacron [veterinary] (TN); SCHEMBL77901; SCHEMBL77902; GTPL569; MLS006010892; Carazolol, analytical standard; 1-(9H-Carbazol-4-yloxy)-3-(isopropylamino)-2-propanol; DTXSID00866648; 1-(9H-carbazol-4-yloxy)-3-; CHEBI:135261; NNNN-TETRAETHYLISOPHTHALAMIDE; BDBM50027663; s6447; AKOS027320502; SB66991; LS-14657; SMR002529709; Carazolol 1000 microg/mL in Acetonitrile; HY-107327; CS-0028150; FT-0601602; 75C298; D07608; T71087; A831600; L000159; Q5037818; 1-(9H-Carbazol-4-yloxy)-3-isopropylamino-propan-2-ol; 1-(9H-Carbazol-4-yloxy)-3-(isopropylamino)-2-propanol #; 1-(9H-Carbazol-4-yloxy)-3-isopropylamino-propan-2-ol(carazolol)
|
|
| CAS | 57775-29-8 | |
| PubChem CID | 71739 | |
| ChEMBL ID | CHEMBL324665 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 298.4 | ALogp: | 3.6 |
| HBD: | 3 | HBA: | 3 |
| Rotatable Bonds: | 6 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 57.3 | Aromatic Rings: | 3 |
| Heavy Atoms: | 22 | QED Weighted: | 0.648 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.138 | MDCK Permeability: | 0.00000988 |
| Pgp-inhibitor: | 0.003 | Pgp-substrate: | 0.988 |
| Human Intestinal Absorption (HIA): | 0.028 | 20% Bioavailability (F20%): | 0.002 |
| 30% Bioavailability (F30%): | 0.008 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.649 | Plasma Protein Binding (PPB): | 81.93% |
| Volume Distribution (VD): | 2.507 | Fu: | 13.26% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.982 | CYP1A2-substrate: | 0.883 |
| CYP2C19-inhibitor: | 0.178 | CYP2C19-substrate: | 0.84 |
| CYP2C9-inhibitor: | 0.019 | CYP2C9-substrate: | 0.56 |
| CYP2D6-inhibitor: | 0.909 | CYP2D6-substrate: | 0.923 |
| CYP3A4-inhibitor: | 0.144 | CYP3A4-substrate: | 0.719 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 10.673 | Half-life (T1/2): | 0.281 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.605 | Human Hepatotoxicity (H-HT): | 0.179 |
| Drug-inuced Liver Injury (DILI): | 0.022 | AMES Toxicity: | 0.103 |
| Rat Oral Acute Toxicity: | 0.66 | Maximum Recommended Daily Dose: | 0.894 |
| Skin Sensitization: | 0.766 | Carcinogencity: | 0.232 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.014 |
| Respiratory Toxicity: | 0.938 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
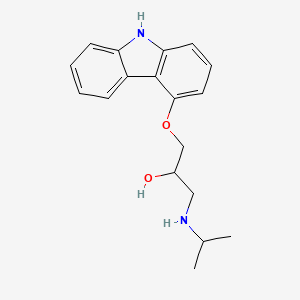
| ENC001521 |  |
0.446 | D04JEE | 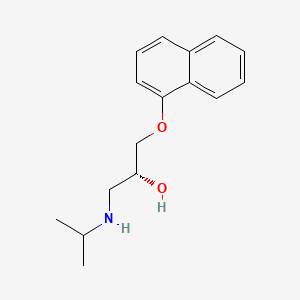 |
0.696 | ||
| ENC000074 | 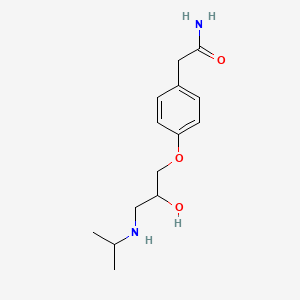 |
0.402 | D0F2PO | 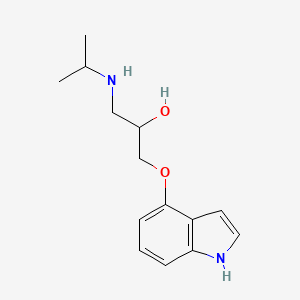 |
0.606 | ||
| ENC002323 | 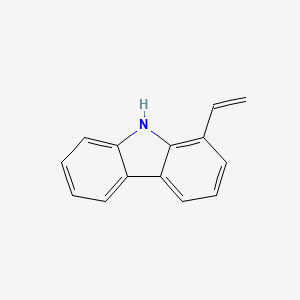 |
0.372 | D0W9LX | 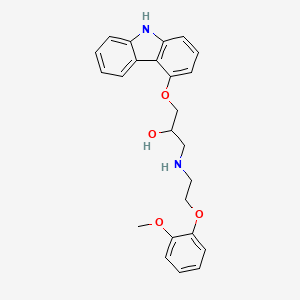 |
0.557 | ||
| ENC005446 | 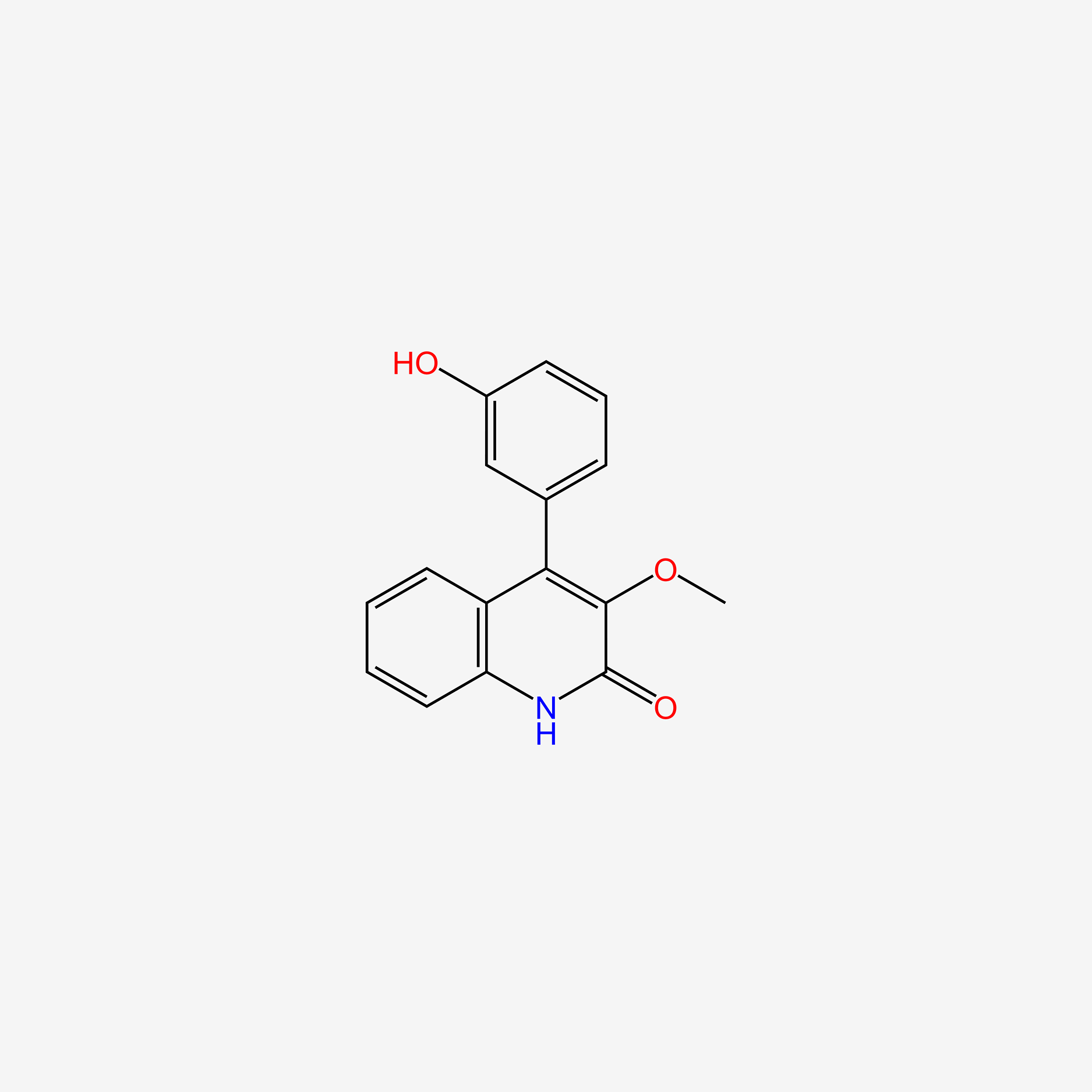 |
0.364 | D0X2MB | 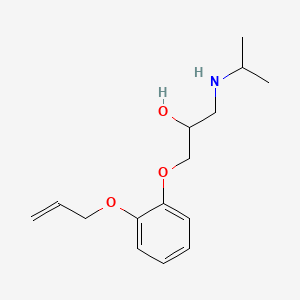 |
0.487 | ||
| ENC000663 | 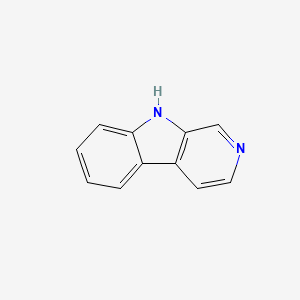 |
0.360 | D0H5MB | 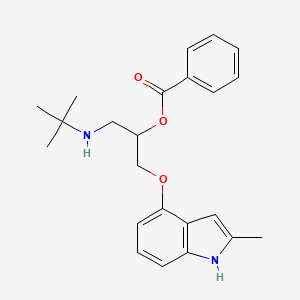 |
0.406 | ||
| ENC004352 | 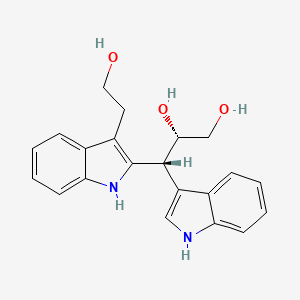 |
0.353 | D01UXC | 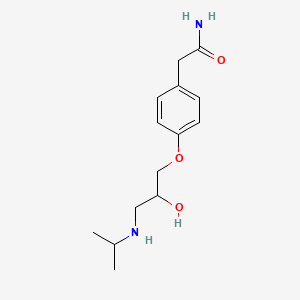 |
0.402 | ||
| ENC004353 | 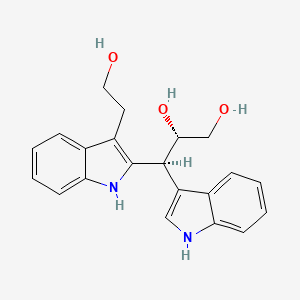 |
0.353 | D0KD1U | 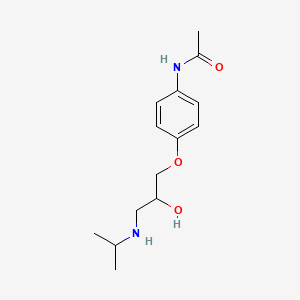 |
0.402 | ||
| ENC004355 | 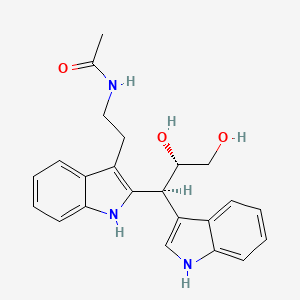 |
0.352 | D0I2MK | 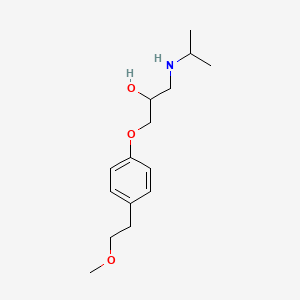 |
0.398 | ||
| ENC004354 |  |
0.352 | D09SSC |  |
0.396 | ||
| ENC000071 |  |
0.347 | D03XTC |  |
0.375 | ||