| InChI |
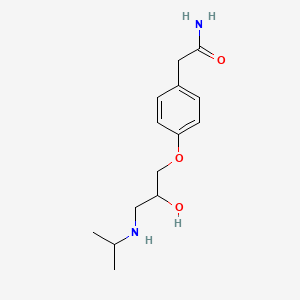
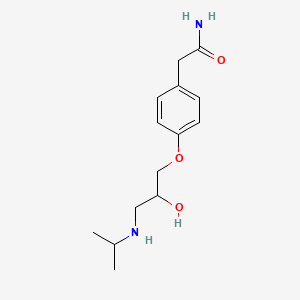
InChI=1S/C14H22N2O3/c1-10(2)16-8-12(17)9-19-13-5-3-11(4-6-13)7-14(15)18/h3-6,10,12,16-17H,7-9H2,1-2H3,(H2,15,18)
|
| Synonyms |
atenolol; 29122-68-7; Tenormin; Blokium; Myocord; Normiten; Prenormine; Tenormine; (RS)-Atenolol; Duraatenolol; Betacard; Corotenol; Tenoblock; Atehexal; Betablok; Cuxanorm; Juvental; Selobloc; Antipressan; Atcardil; Atenblock; Evitocor; Farnormin; Internolol; Normalol; Premorine; Prenolol; Tenoprin; Tensimin; Vascoten; Vericordin; Alinor; Anselol; Atecard; Atendol; Atenet; Atenil; Atereal; Aterol; Hipres; Hypoten; Ibinolo; Lotenal; Oraday; Serten; Stermin; Tenidon; Tenolol; Tredol; Uniloc; Wesipin; Altol; Ateni; Noten; Xaten; Seles beta; Apo-Atenolol; Felo-Bits; Lo-ten; Atenolin; Atenomel; Blocotenol; Cardaxen; Cardiopress; Jenatenol; Panapres; Plenacor; Servitenol; Tenobloc; Aircrit; Betasyn; Ormidol; Prinorm; Unibloc; Loten; Atenol acis; Atenol Cophar; Atenol Fecofar; Atenol Heumann; Atenol Nordic; Atenol Quesada; Atenol Gador; Atenol Stada; Atenol-Mepha; Atenol-Wolff; Atenol Atid; Atenol ct; Atenol Tika; Atenol Trom; Atenol Genericon; Betatop Ge; Atenol von ct; Atenol-ratiopharm; Atenol AL; Atenol PB; Atenol GNR; Atenol MSD; Atenol NM Pharma; Scheinpharm Atenol; Atenol 1A pharma; Atenololum; 2-(4-(2-hydroxy-3-(isopropylamino)propoxy)phenyl)acetamide; Tenormine [French]; (r,s)-atenolol; Atenololum [INN-Latin]; ICI 66082; 2-[4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl]acetamide; Tenormin (TN); ICI-66082; Novaten; 1-p-Carbamoylmethylphenoxy-3-isopropylamino-2-propanol; ICI 66,082; 2-[4-(2-Hydroxy-3-isopropylaminopropoxy)phenyl]acetamide; 2-(p-(2-Hydroxy-3-(isopropylamino)propoxy)phenyl)acetamide; 4-(2-Hydroxy-3-((1-methylethyl)amino)propoxy)benzeneacetamide; 2-(4-{2-hydroxy-3-[(propan-2-yl)amino]propoxy}phenyl)acetamide; Benzeneacetamide, 4-[2-hydroxy-3-[(1-methylethyl)amino]propoxy]-; C07AB03; CHEMBL24; duratenol; NSC-757832; Benzeneacetamide, 4-(2-hydroxy-3-((1-methylethyl)amino)propoxy)-; 50VV3VW0TI; MLS000069622; CHEBI:2904; Atenolol Bp; 60966-51-0; SMR000036768; Atenol; 2-{4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl}acetamide; Acetamide, 2-(p-(2-hydroxy-3-(isopropylamino)propoxy)phenyl)-; Teno-basan; Neatenol; Tensotin; Atcard; dl-Atenolol; CCRIS 4196; (+/-)-Atenolol; HSDB 6526; SR-01000000159; EINECS 249-451-7; EINECS 262-544-7; MFCD00057645; UNII-50VV3VW0TI; BRN 2739235; 2-(4-[2-Hydroxy-3-(isopropylamino)propoxy]phenyl)acetamide; Artrenolol; 2-(4-(2-Hydroxy-3-(Isopropylamino)propoxy)phenyl)ethanamide; 4-(2-Hydroxy-3-[(1-methylethyl)amino]propoxy)benzeneacetamide; 2-(4-(2-Hydroxy-3-isopropylaminopropoxy)phenyl)acetamid; phenyl)acetamide; Atenolol [USAN:BAN:INN:JAN]; (y)-Atenolol; Atenalol (RS); Atenolol,(S); (?)-Atenolol; Atenolol [USAN:USP:INN:BAN:JAN]; (A+/-)-Atenolol; Tenoretic (Salt/Mix); Atenolol (JAN/USP); Spectrum_001364; ATENOLOL [HSDB]; ATENOLOL [USAN]; ATENOLOL [INN]; ATENOLOL [JAN]; ATENOLOL [MI]; ATENOLOL [VANDF]; Opera_ID_1283; Spectrum2_001411; Spectrum3_001448; Spectrum4_000435; Spectrum5_001509; ATENOLOL [MART.]; DSSTox_CID_2628; ATENOLOL [USP-RS]; ATENOLOL [WHO-DD]; ATENOLOL [WHO-IP]; A 7655; SCHEMBL4362; DSSTox_RID_76663; DSSTox_GSID_22628; Lopac0_000121; Oprea1_448775; BSPBio_002915; GTPL548; KBioGR_000790; KBioSS_001844; MLS001066372; MLS001074163; MLS001304038; DivK1c_000057; SPECTRUM1501127; Atenolol (JP17/USP/INN); SPBio_001482; ATENOLOL [ORANGE BOOK]; ATENOLOL [EP MONOGRAPH]; ATENOLOL [USP IMPURITY]; ATENOLOL [USP MONOGRAPH]; DTXSID2022628; BDBM25753; HMS500C19; KBio1_000057; KBio2_001844; KBio2_004412; KBio2_006980; KBio3_002415; METKIMKYRPQLGS-UHFFFAOYSA-; ATENOLOLUM [WHO-IP LATIN]; NINDS_000057; S-Atenolol-D7 (Iso-propyl-d7); 2-{4-[2-Hydroxy-3-(isopropylamino)propoxy]-phenyl}acetamide; HMS1569L13; HMS1921H09; HMS2090I19; HMS2092D19; HMS2233E06; HMS3259K08; HMS3260I04; HMS3266K13; HMS3369B14; HMS3369D20; HMS3369P20; HMS3411G21; HMS3675G21; HMS3886G03; Pharmakon1600-01501127; ( inverted question mark)-Atenolol; TENORETIC COMPONENT ATENOLOL; BCP12899; Atenolol 1.0 mg/ml in Acetonitrile; Atenolol, >=98% (TLC), powder; Tox21_302426; Tox21_500121; 2-[4-({2-hydroxy-3-[(1-methylethyl)amino]propyl}oxy)phenyl]acetamide; BBL009276; CCG-39010; GEO-03413; NSC757832; s4817; STK528649; AKOS005111050; ATENOLOL COMPONENT OF TENORETIC; AC-8245; Atenolol, analytical reference material; DB00335; KS-5341; LP00121; NC00548; NSC 757832; SDCCGSBI-0050109.P004; IDI1_000057; MRF-0000571; NCGC00015007-06; NCGC00015007-07; NCGC00015007-08; NCGC00015007-09; NCGC00015007-10; NCGC00015007-11; NCGC00015007-13; NCGC00015007-24; NCGC00024566-03; NCGC00024566-04; NCGC00024566-05; NCGC00024566-06; NCGC00024566-07; NCGC00255122-01; NCGC00260806-01; BA166036; HY-17498; SBI-0050109.P003; CAS-29122-68-7; DB-072177; DB-079552; EU-0100121; FT-0662315; FT-0662316; FT-0693045; 2-(4-(2-hydroxy-3-(isopropylamino)propoxy); BIM-0050109.0001; D00235; EN300-119532; O10469; AB00052208-13; AB00052208-15; AB00052208_16; 122A687; L000116; Q411325; Q-200656; SR-01000000159-2; SR-01000000159-4; SR-01000000159-5; SR-01000000159-8; BRD-A20239487-001-02-5; BRD-A20239487-001-15-7; Atenolol, European Pharmacopoeia (EP) Reference Standard; Z1521553991; Atenolol, United States Pharmacopeia (USP) Reference Standard; (+)-4-[2-Hydroxy-3-[(1-methylethyl)amino]propoxy]benzeneacetamide; (RS)-4-[2-Hydroxy-3-[(1-methylethyl)amino]propoxy]benzeneacetamide; 2-(P-(HYDROXY-3-(ISOPROPYLAMINO)PROPOXY)PHENYL)ACETAMIDE; (+/-)-4-(2-Hydroxy-3-[(1-methylethyl)amino]propoxy)benzeneacetamide; 2-(4-{[(2S)-2-hydroxy-3-(propan-2-ylamino)propyl]oxy}phenyl)acetamide; Atenolol, Pharmaceutical Secondary Standard; Certified Reference Material; 2-(P-(2-HYDROXY-3-(ISOPROPYLAMINO)PROPOXY)PHENYL)ACETAMIDE (RACEMATE); 2-[4-({(2R)-2-hydroxy-3-[(1-methylethyl)amino]propyl}oxy)phenyl]acetamide; 106020-65-9
|