NPs Basic Information
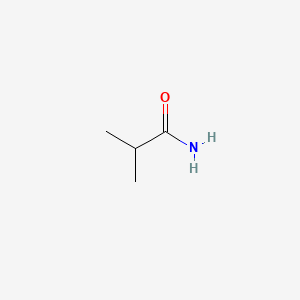
|
Name |
Isobutyramide
|
| Molecular Formula | C4H9NO | |
| IUPAC Name* |
2-methylpropanamide
|
|
| SMILES |
CC(C)C(=O)N
|
|
| InChI |
InChI=1S/C4H9NO/c1-3(2)4(5)6/h3H,1-2H3,(H2,5,6)
|
|
| InChIKey |
WFKAJVHLWXSISD-UHFFFAOYSA-N
|
|
| Synonyms |
Isobutyramide; 563-83-7; 2-Methylpropanamide; 2-Methylpropionamide; Propanamide, 2-methyl-; Isobutyrimidic acid; Isobutylamide; MFCD00008019; 82UOE7B38Z; NSC-8423; dimethylacetoamide; NSC 8423; EINECS 209-265-9; BRN 1737615; UNII-82UOE7B38Z; C-isopropylformamide; 2-methyl-propanamide; isobutyric acid amide; Isobutyramide, 99%; CRYSTALPONCEAU6R; 1-carbamoyl-1-methylethyl; 4-02-00-00852 (Beilstein Handbook Reference); 68424-61-3; CHEMBL352219; Glycerides, C16-18 and C18-unsatd. mono- and di-; DTXSID1060340; NSC8423; CHEBI:193555; ZINC1484944; BDBM50224866; AKOS001084432; CS-W019979; NCI60_041854; SY015332; DB-052904; DB-072139; FT-0627380; FT-0672107; I0102; EN300-17833; W-105521; Q10859786; Z57046209; IBO
|
|
| CAS | 563-83-7 | |
| PubChem CID | 68424 | |
| ChEMBL ID | CHEMBL352219 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 87.12 | ALogp: | 0.2 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 43.1 | Aromatic Rings: | 0 |
| Heavy Atoms: | 6 | QED Weighted: | 0.498 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.556 | MDCK Permeability: | 0.00021824 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.419 |
| Human Intestinal Absorption (HIA): | 0.004 | 20% Bioavailability (F20%): | 0.005 |
| 30% Bioavailability (F30%): | 0.002 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.997 | Plasma Protein Binding (PPB): | 21.58% |
| Volume Distribution (VD): | 0.958 | Fu: | 76.04% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.221 | CYP1A2-substrate: | 0.258 |
| CYP2C19-inhibitor: | 0.03 | CYP2C19-substrate: | 0.587 |
| CYP2C9-inhibitor: | 0.007 | CYP2C9-substrate: | 0.162 |
| CYP2D6-inhibitor: | 0.009 | CYP2D6-substrate: | 0.281 |
| CYP3A4-inhibitor: | 0.008 | CYP3A4-substrate: | 0.249 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 8.033 | Half-life (T1/2): | 0.32 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.011 | Human Hepatotoxicity (H-HT): | 0.06 |
| Drug-inuced Liver Injury (DILI): | 0.042 | AMES Toxicity: | 0.032 |
| Rat Oral Acute Toxicity: | 0.026 | Maximum Recommended Daily Dose: | 0.019 |
| Skin Sensitization: | 0.125 | Carcinogencity: | 0.034 |
| Eye Corrosion: | 0.072 | Eye Irritation: | 0.943 |
| Respiratory Toxicity: | 0.021 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000149 | 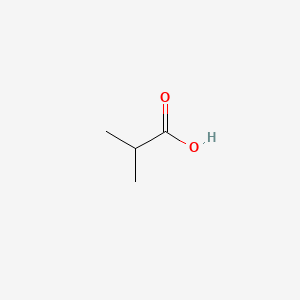 |
0.529 | D09PUL | 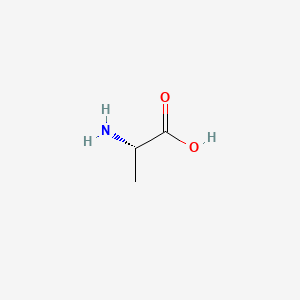 |
0.368 | ||
| ENC000376 | 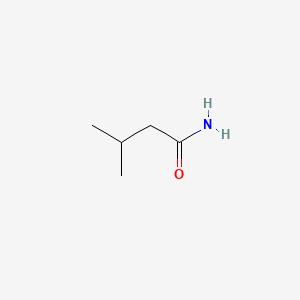 |
0.500 | D02XBW | 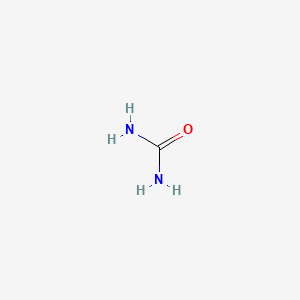 |
0.313 | ||
| ENC000382 | 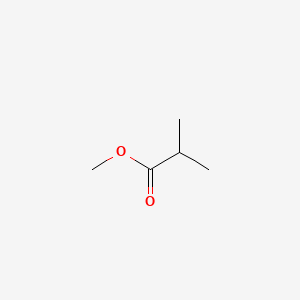 |
0.429 | D07ZTO | 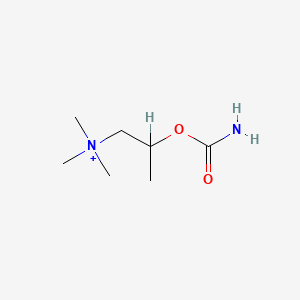 |
0.290 | ||
| ENC000186 | 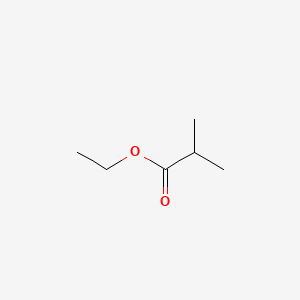 |
0.375 | D00ZOF | 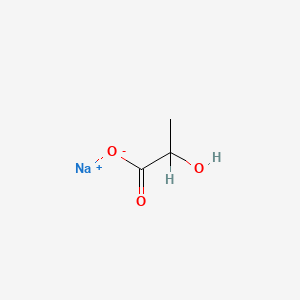 |
0.286 | ||
| ENC000010 | 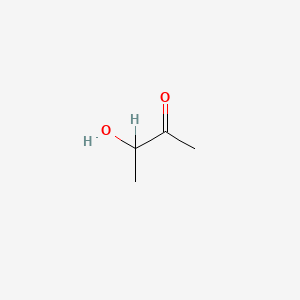 |
0.368 | D08QGD | 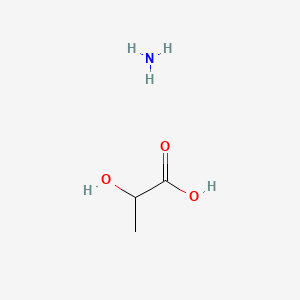 |
0.286 | ||
| ENC000237 | 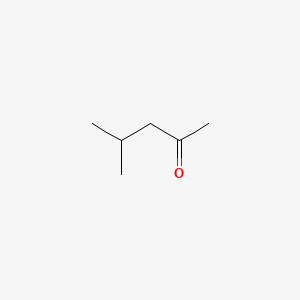 |
0.364 | D00WUF | 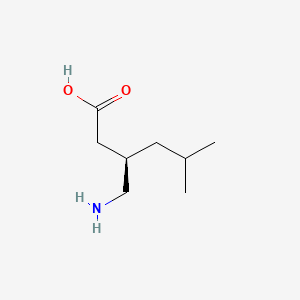 |
0.281 | ||
| ENC000351 | 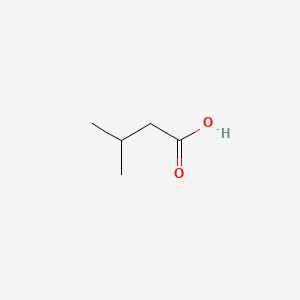 |
0.364 | D0ZK8H | 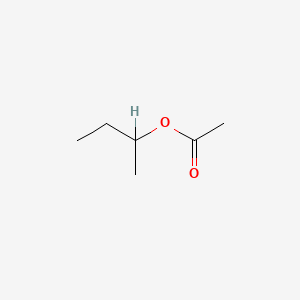 |
0.269 | ||
| ENC000771 | 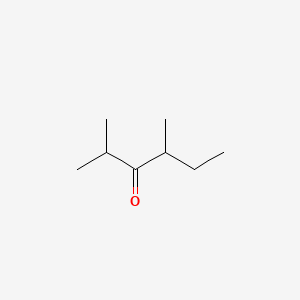 |
0.346 | D01BQK | 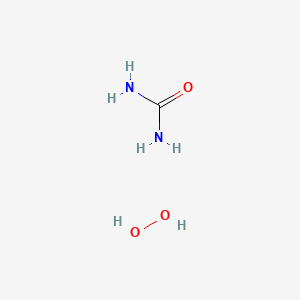 |
0.263 | ||
| ENC000824 | 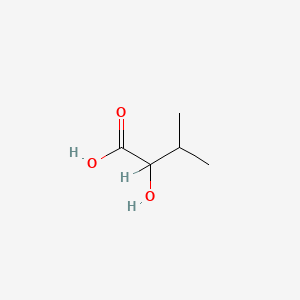 |
0.333 | D07CWD | 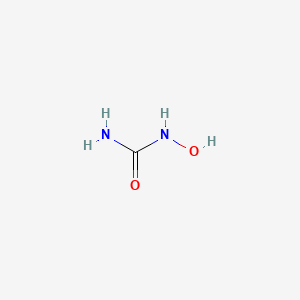 |
0.263 | ||
| ENC000246 | 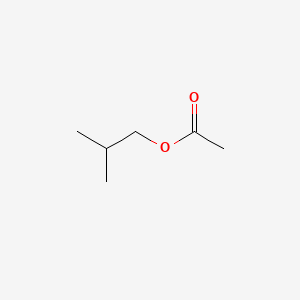 |
0.320 | D08HZC | 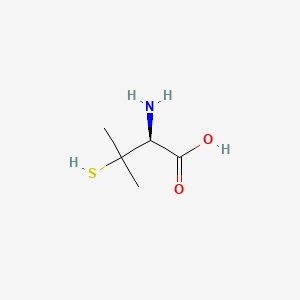 |
0.259 | ||