| Synonyms |
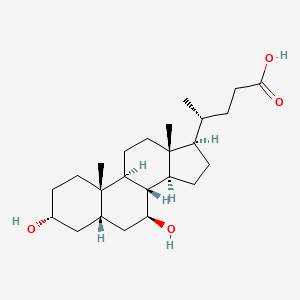
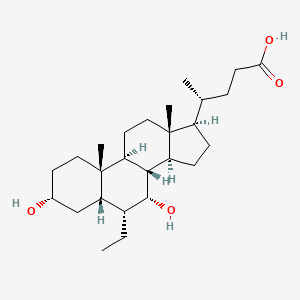
URSODEOXYCHOLIC ACID; ursodiol; 128-13-2; Actigall; ursodeoxycholate; UDCA; Ursofalk; Ursolvan; UrSO; Delursan; Ursodesoxycholic acid; Urso Forte; Destolit; Ursochol; Cholit-ursan; Litursol; Solutrat; Ursobilin; Ursodamor; Arsacol; Deursil; Lyeton; Ursacol; Urso DS; UDCS; 3alpha,7beta-Dihydroxy-5beta-cholan-24-oic acid; Ursosan; Urso 250; 3-alpha,7-beta-Dioxycholanic acid; Ursocholic acid, deoxy-; Ursodeoxycholicacid; 3-alpha,7-beta-Dihydroxycholanic acid; Ursonorm; NSC 683769; (3alpha,5beta,7beta)-3,7-dihydroxycholan-24-oic acid; 3-alpha,7-beta-Dihydroxy-5-beta-cholanoic acid; NSC 657950; BRN 3219888; 3,7-Dihydroxycholan-24-oic acid; (4R)-4-[(3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoic acid; Peptarom; Ursodeoxycholic acid [INN]; CHEMBL1551; MLS000028461; CHEBI:9907; 724L30Y2QR; NSC-683769; Ursodiol [USAN]; Antigall; SMR000058403; Urosiol; Cholan-24-oic acid, 3,7-dihydroxy-, (3.alpha.,5.beta.,7.beta.)-; 7-beta-Hydroxylithocholic acid; Ursodexycholic Acid; Acido ursodeossicolico [Italian]; (R)-4-((3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoic acid; Acido ursodeoxicolico; Acido ursodeossicolico; Acido ursodeoxicolico [INN-Spanish]; Acidum ursodeoxycholicum [INN-Latin]; Acide ursodesoxycholique; Acide ursodesoxycholique [INN-French]; Acidum ursodeoxycholicum; Actigall (TN); Ursodiol (USP); CCRIS 5502; Urso (TN); SR-01000737091; Ursodiol [USAN:USP]; EINECS 204-879-3; MFCD00003680; Ursodexycholate; Paptarom; Udiliv; Desol; Urdes; UNII-724L30Y2QR; Urosdesoxycholate; 17-beta-(1-Methyl-3-carboxypropyl)etiocholane-3-alpha,7-beta-diol; Ursodeoxycholoc acid; Urosdesoxycholic acid; 5beta-Cholanic Acid-3alpha,7beta-diol; URSODIOL [INCI]; Cholan-24-oic acid, 3,7-dihydroxy-, (3alpha,5beta,7beta)-; 3alpha,7beta-Dihydroxy-5beta-cholanic acid; URSODIOL [MI]; Ursodiol (Actigal Urso); URSODIOL [VANDF]; Prestwick0_000958; Prestwick1_000958; Prestwick2_000958; Prestwick3_000958; U0030; 7A-Hydroxylithocholic acid; URSODIOL [USP-RS]; EC 204-879-3; U-9000; SCHEMBL27200; BSPBio_000956; 4-10-00-01604 (Beilstein Handbook Reference); 7bet.-Hydroxylithocholic acid; cid_31401; MLS001066373; MLS002548885; SPBio_003105; Ursodeoxycholic acid, >=99%; URSODIOL [ORANGE BOOK]; BPBio1_001052; GTPL7104; 7.beta.-Hydroxylithocholic acid; Ursodiol (Ursodeoxycholic Acid); 5-beta-Cholan-24-oic acid, 3-alpha,7-beta-dihydroxy-; DTXSID6023731; URSODIOL [USP MONOGRAPH]; BDBM53721; (3alpha,5beta,7beta,8xi)-3,7-dihydroxycholan-24-oic acid; HMS1570P18; HMS2097P18; HMS2233L14; HMS3259A13; HMS3714P18; Ursodeoxycholic acid (JP17/INN); URSODEOXYCHOLIC ACID [JAN]; 3alpha,7beta-dihydroxycholanic acid; ACT02676; Cholan-24-oic acid, 3,7-dihydroxy-, (3-alpha,5-beta,7-beta)-; ZINC3914809; 3a,7b-dihydroxy-5b-cholan-24-oate; LMST04010033; s1643; URSODEOXYCHOLIC ACID [MART.]; URSODEOXYCHOLIC ACID [WHO-DD]; 5A-Cholan-24-oic acid-3A,7A-diol; AKOS015955898; CCG-220958; CS-1932; DB01586; KS-5243; NC00487; SMP2_000012; 3.alpha.,7.beta.-Dihydroxycholanic acid; 3A,7A-Dihydroxy-5A-holan-24-oic acid; 3a,7b-dihydroxy-5b-cholan-24-oic acid; NCGC00179363-01; NCGC00179363-12; AC-18919; CAS#128-13-2; CPD000058403; HY-13771; NCI60_028904; URSODEOXYCHOLIC ACID [EP IMPURITY]; URSODEOXYCHOLIC ACID [EP MONOGRAPH]; AB00513977; (3a,5b,7b)-3,7-dihydroxy-cholan-24-oate; 3alpha, 7beta-dihydroxy-5beta-cholanoic acid; 5bet.-Cholan-24-oic acid-3alp.,7bet.-diol; (3a,5b,7b)-3,7-dihydroxycholan-24-oic acid; C07880; D00734; EN300-373707; ((3a,5b,7b)-3,7-Dihydroxycholan-24-oic acid; (3a,5b,7b)-3,7-dihydroxy-cholan-24-oic acid; AB00513977-09; AB00513977_10; 3alp.,7bet.-Dihydroxy-5bet.-cholan-24-oic acid; A905413; Q241374; 3.alpha.,7.beta.-Dihydroxy-5.beta.-cholanic acid; J-005566; J-650210; SR-01000737091-3; SR-01000737091-4; BRD-K15697815-001-16-2; 3.alpha.,7.beta.-Dihydroxy-5.beta.-cholan-24-oic acid; Z2588039022; 3.ALPHA.,7.BETA.-DIHYDROXY-5B-CHOLAN-24-OIC ACID; 5.beta.-Cholan-24-oic acid, 3.alpha.,7.beta.-dihydroxy-; Ursodiol, United States Pharmacopeia (USP) Reference Standard; Ursodeoxycholic acid, British Pharmacopoeia (BP) Reference Standard; Ursodeoxycholic acid, European Pharmacopoeia (EP) Reference Standard; 17.beta.-(1-Methyl-3-carboxypropyl)etiocholane-3.alpha.,7.beta.-diol; 3,7-Dihydroxycholan-24-oic acid-, (3.alpha.,5.beta.,7.beta.)- #; Ursodeoxycholic acid, 500 mug/mL in methanol, certified reference material; Ursodiol, Pharmaceutical Secondary Standard; Certified Reference Material; Ursodeoxycholic acid for system suitability, European Pharmacopoeia (EP) Reference Standard; (4R)-4-[(1R,3aS,3bR,4S,5aS,7R,9aS,9bS,11aR)-4,7-dihydroxy-9a,11a-dimethyl-hexadecahydro-1H-cyclopenta[a]phenanthren-1-yl]pentanoic acid; (4R)-4-[(1S,2S,5R,7S,9S,10R,11S,14R,15R)-5,9-dihydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-14-yl]pentanoic acid; (4R)-4-[(1S,2S,5R,7S,9S,10R,11S,14R,15R)-5,9-Dihydroxy-2,15-dimethyltetracyclo[8.7.0.02,1.011,1]heptadecan-14-yl]pentanoic acid; (4R)-4-[(3R,5S,7S,8R,9S,10S,13R,14S,17R)-10,13-dimethyl-3,7-bis(oxidanyl)-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoic acid; (4R)-4-[(3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoicacid; (4R)-4-[(3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]valeric acid; 108609-27-4
|