NPs Basic Information
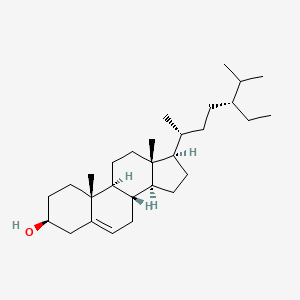
|
Name |
Beta-Sitosterol
|
| Molecular Formula | C29H50O | |
| IUPAC Name* |
(3S,8S,9S,10R,13R,14S,17R)-17-[(2R,5R)-5-ethyl-6-methylheptan-2-yl]-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol
|
|
| SMILES |
CC[C@H](CC[C@@H](C)[C@H]1CC[C@@H]2[C@@]1(CC[C@H]3[C@H]2CC=C4[C@@]3(CC[C@@H](C4)O)C)C)C(C)C
|
|
| InChI |
InChI=1S/C29H50O/c1-7-21(19(2)3)9-8-20(4)25-12-13-26-24-11-10-22-18-23(30)14-16-28(22,5)27(24)15-17-29(25,26)6/h10,19-21,23-27,30H,7-9,11-18H2,1-6H3/t20-,21-,23+,24+,25-,26+,27+,28+,29-/m1/s1
|
|
| InChIKey |
KZJWDPNRJALLNS-VJSFXXLFSA-N
|
|
| Synonyms |
BETA-SITOSTEROL; 83-46-5; Sitosterol; Cupreol; Azuprostat; 22,23-Dihydrostigmasterol; Quebrachol; Triastonal; Cinchol; Harzol; Rhamnol; beta-Sitosterin; Nimbosterol; B-Sitosterol; (-)-beta-Sitosterol; alpha-Dihydrofucosterol; Prostasal; Sito-Lande; 24alpha-Ethylcholesterol; (24R)-Ethylcholest-5-en-3beta-ol; (3beta)-Stigmast-5-en-3-ol; Betaprost; Sobatum; beta Sitosterol; alpha-Phytosterol; (24R)-Stigmast-5-en-3beta-ol; Angelicin (steroid); SKF 14463; Beta-sistosterol; Stigmast-5-en-3beta-ol; a-Dihydrofucosterol; Phytosterol; CHEBI:27693; NSC8096; SITOSTEROL, BETA; Stigmast-5-en-3-ol, (3beta)-; .alpha.-Dihydrofucosterol; CHEMBL221542; S347WMO6M4; .beta.-Sitosterol; NSC-8096; NSC49083; .alpha.-Phytosterol; Sitosterol, .beta.; NSC 18173; NSC-18173; NSC-49083; Stigmasterol,23-dihydro-; Stigmasterol, 22,23-dihydro-; sitosterin; .beta.-Sitosterin; NSC 8096; SITOSTEROL, BETA-; Stigmast-5-en-3-ol, (3.beta.)-; (3S,8S,9S,10R,13R,14S,17R)-17-[(2R,5R)-5-ethyl-6-methylheptan-2-yl]-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol; 24.alpha.-Ethylcholesterol; Stigmast-5-en-3-ol, (3b)-; Stigmast-5-en-3.beta.-ol; SR-05000002307; 24-alpha-Ethylcholesterol; Stigmast-5-en-3-beta-ol; UNII-S347WMO6M4; delta5-Stigmasten-3-beta-ol; Sitosterol beta; a-Phytosterol; b-Sitosterin; beta-Phytosterol; CCRIS 5529; ss--Sitosterol; .beta.Sitosterin; .beta.Sitosterol; beta -Sitosterol; (3-beta)-Stigmast-5-en-3-ol; CAS-83-46-5; NCGC00095716-01; (3S,8S,9S,10R,13R,14S,17R)-17-((2R,5R)-5-ethyl-6-methylheptan-2-yl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-ol; (3S,8S,9S,10R,13R,14S,17R)-17-[(1R,4R)-4-ethyl-1,5-dimethyl-hexyl]-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol; EINECS 201-480-6; (-)-b-Sitosterol; Harzol (TN); MFCD00003631; 24a-Ethylcholesterol; alpha.Dihydrofucosterol; D5-Stigmasten-3b-ol; Stigmast-5-en-3b-ol; AI3-26020; Prestwick0_000985; Prestwick1_000985; Prestwick2_000985; Prestwick3_000985; Delta5-Stigmasten-3b-ol; stigmast-5-ene-3beta-ol; DSSTox_CID_2481; beta-Sitosterol, >=70%; Beta-sitosterol [WHO-DD]; SITOSTEROL [MART.]; beta-Sitosterol (Synthetic); DSSTox_RID_76601; BIDD:PXR0121; DSSTox_GSID_22481; SCHEMBL16105; 22,23-dihydro-Stigmasterol; BSPBio_001049; BIDD:ER0636; SPBio_002950; BETA-SITOSTEROL [INCI]; BPBio1_001155; MEGxp0_001710; BETA SITOSTEROL [VANDF]; .BETA.-SITOSTEROL [MI]; DTXSID5022481; (24R)-Stigmast-5-en-3b-ol; ACon1_000287; BETA-SITOSTEROL [USP-RS]; CHEBI:176889; HMS1571E11; HMS2098E11; (24R)-Ethylcholest-5-en-3b-ol; 24-Ethylcholest-5-en-3.beta.-ol; NSC18173; ZINC4095717; beta-Sitosterol, analytical standard; Tox21_111514; BDBM50218197; beta-Sitosterol, synthetic, >=95%; LMST01040129; NSC821067; s2273; AKOS005267194; CCG-208334; DB14038; NSC-821067; beta-Sitosterol, from soybean, >=96%; SMP1_000274; NCGC00142598-02; NCGC00142598-03; (3.BETA.)-STIGMAST-5-EN-3-OL; 14-((1S,4R)-4-ethyl-1,5-dimethylhexyl)(1S,5S,10S,11S,2R,14R,15R)-2,15-dimethyl tetracyclo[8.7.0.0<2,7>.0<11,15>]heptadec-7-en-5-ol; AC-24183; NCI60_041777; AB00513984; S0040; .DELTA.(SUP 5)-STIGMASTEN-3.BETA.-OL; C01753; D08518; A840577; alpha-Dihydrofucosterol, 22,23-Dihydrostigmasterol; Q-200712; SR-05000002307-2; SR-05000002307-3; Q63409374; .BETA.-SITOSTEROL (CONSTITUENT OF PYGEUM) [DSC]; BETA-SITOSTEROL (CONSTITUENT OF SAW PALMETTO) [DSC]; beta-Sitosterol, analytical standard, from soybean, >=40%; beta-Sitosterol, primary pharmaceutical reference standard; 24.BETA.-ETHYL-.DELTA.(SUP 5)-CHOLESTEN-3.BETA.-OL; BETA-SITOSTEROL (CONSTITUENT OF STINGING NETTLE) [DSC]; Beta-sitosterol, European Pharmacopoeia (EP) Reference Standard; beta-Sitosterol, United States Pharmacopeia (USP) Reference Standard; beta-Sitosterol, certified reference material, 100 mug/mL in chloroform; (3S,9S,10R,13R,14S,17R)-17-((2R,5R)-5-ethyl-6-methylheptan-2-yl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-ol; 17-(5-ethyl-6-methyl-heptan-2-yl)-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol; 76772-70-8
|
|
| CAS | 83-46-5 | |
| PubChem CID | 222284 | |
| ChEMBL ID | CHEMBL221542 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 414.7 | ALogp: | 9.3 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 6 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 20.2 | Aromatic Rings: | 4 |
| Heavy Atoms: | 30 | QED Weighted: | 0.416 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.679 | MDCK Permeability: | 0.00001070 |
| Pgp-inhibitor: | 0.972 | Pgp-substrate: | 0.092 |
| Human Intestinal Absorption (HIA): | 0.002 | 20% Bioavailability (F20%): | 0.889 |
| 30% Bioavailability (F30%): | 0.054 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.136 | Plasma Protein Binding (PPB): | 93.38% |
| Volume Distribution (VD): | 1.484 | Fu: | 1.30% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.095 | CYP1A2-substrate: | 0.526 |
| CYP2C19-inhibitor: | 0.143 | CYP2C19-substrate: | 0.923 |
| CYP2C9-inhibitor: | 0.208 | CYP2C9-substrate: | 0.131 |
| CYP2D6-inhibitor: | 0.142 | CYP2D6-substrate: | 0.344 |
| CYP3A4-inhibitor: | 0.538 | CYP3A4-substrate: | 0.731 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.21 | Half-life (T1/2): | 0.049 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.875 | Human Hepatotoxicity (H-HT): | 0.131 |
| Drug-inuced Liver Injury (DILI): | 0.551 | AMES Toxicity: | 0.002 |
| Rat Oral Acute Toxicity: | 0.003 | Maximum Recommended Daily Dose: | 0.214 |
| Skin Sensitization: | 0.963 | Carcinogencity: | 0.12 |
| Eye Corrosion: | 0.943 | Eye Irritation: | 0.869 |
| Respiratory Toxicity: | 0.464 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001107 | 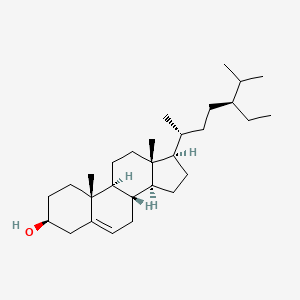 |
1.000 | D0Y7LD | 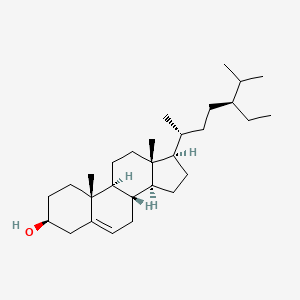 |
1.000 | ||
| ENC000961 |  |
0.852 | D0B4RU |  |
0.581 | ||
| ENC000125 |  |
0.809 | D0K0EK |  |
0.495 | ||
| ENC001647 |  |
0.794 | D0M4WA | 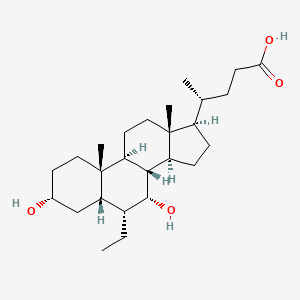 |
0.422 | ||
| ENC001545 | 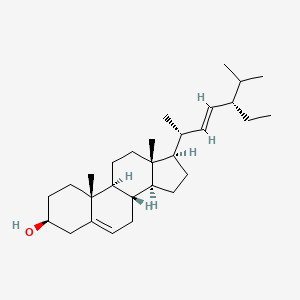 |
0.729 | D0G3SH | 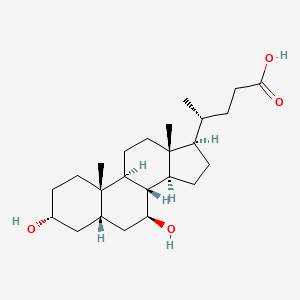 |
0.416 | ||
| ENC001170 | 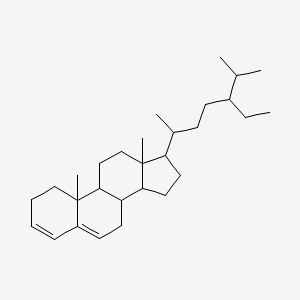 |
0.708 | D03ZTE | 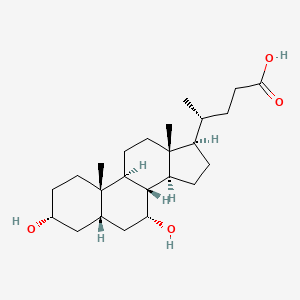 |
0.416 | ||
| ENC004758 | 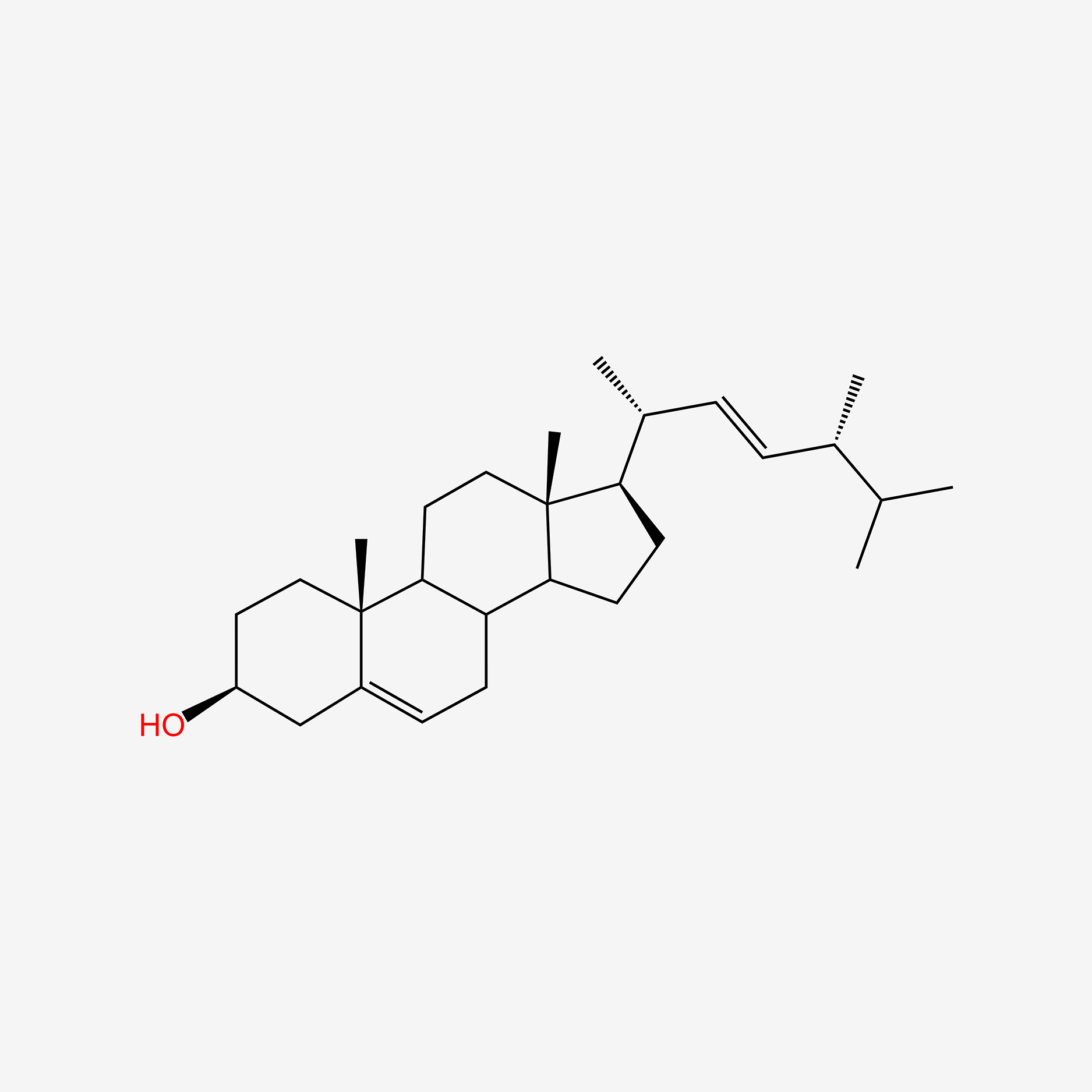 |
0.698 | D02STN | 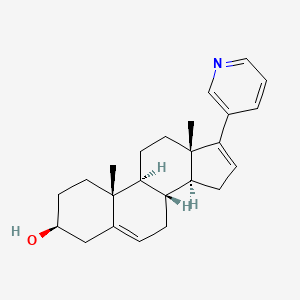 |
0.398 | ||
| ENC001558 | 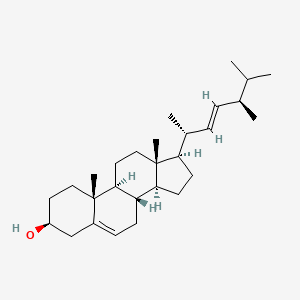 |
0.698 | D06XMU | 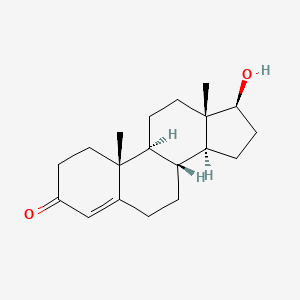 |
0.392 | ||
| ENC002882 | 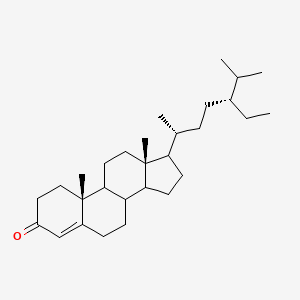 |
0.694 | D0K5WS |  |
0.364 | ||
| ENC001764 | 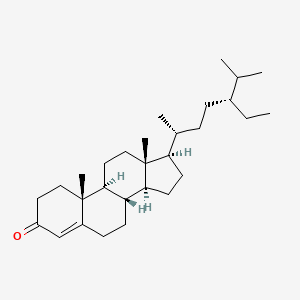 |
0.694 | D07BSQ | 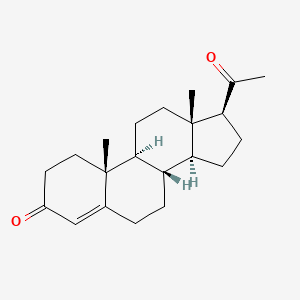 |
0.361 | ||