NPs Basic Information
|
Name |
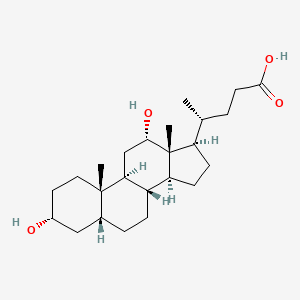
Deoxycholic Acid
|
| Molecular Formula | C24H40O4 | |
| IUPAC Name* |
(4R)-4-[(3R,5R,8R,9S,10S,12S,13R,14S,17R)-3,12-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoic acid
|
|
| SMILES |
C[C@H](CCC(=O)O)[C@H]1CC[C@@H]2[C@@]1([C@H](C[C@H]3[C@H]2CC[C@H]4[C@@]3(CC[C@H](C4)O)C)O)C
|
|
| InChI |
InChI=1S/C24H40O4/c1-14(4-9-22(27)28)18-7-8-19-17-6-5-15-12-16(25)10-11-23(15,2)20(17)13-21(26)24(18,19)3/h14-21,25-26H,4-13H2,1-3H3,(H,27,28)/t14-,15-,16-,17+,18-,19+,20+,21+,23+,24-/m1/s1
|
|
| InChIKey |
KXGVEGMKQFWNSR-LLQZFEROSA-N
|
|
| Synonyms |
DEOXYCHOLIC ACID; 83-44-3; deoxycholate; Desoxycholic acid; Cholerebic; Cholorebic; Choleic acid; Degalol; Deoxycholatic acid; Droxolan; Pyrochol; Septochol; Desoxycholsaeure; 7alpha-Deoxycholic acid; 3alpha,12alpha-Dihydroxy-5beta-cholanic acid; Cholic acid, deoxy-; Dihydroxycholanoic acid; ATX-101; 7-Deoxycholic acid; Kybella; (3alpha,5beta,12alpha)-3,12-Dihydroxycholan-24-oic acid; 3,12-Dihydroxycholanic acid; Deoxy cholic acid; 3alpha,12alpha-Dihydroxy-5beta-cholan-24-oic acid; 5-beta-Deoxycholic acid; Desoxycholic acid [NF]; Desoxycholsaeure [German]; (4R)-4-[(3R,5R,8R,9S,10S,12S,13R,14S,17R)-3,12-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoic acid; NSC8797; NSC 8797; NSC-8797; MFCD00003673; (R)-4-((3R,5R,8R,9S,10S,12S,13R,14S,17R)-3,12-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoic acid; 7.alpha.-Deoxycholic acid; 3.alpha.,12.alpha.-Dihydroxycholanic acid; DWJ211; CHEBI:28834; Cholan-24-oic acid, 3,12-dihydroxy-, (3alpha,5beta,12alpha)-; DWJ-211; 005990WHZZ; 3-alpha,12-alpha-Dihydroxycholansaeure [German]; 3-alpha,12-alpha-Dihydroxy-5-beta-cholanoic acid; ATX-101 (DEOXYCHOLIC ACID); 5beta-Cholan-24-oic acid, 3alpha,12alpha-dihydroxy-; 3-alpha,12-alpha-Dihydroxy-5-beta-cholan-24-oic acid; Cholan-24-oic acid, 3,12-dihydroxy-, (3a,5b,12a)-; Cholan-24-oic, 3,12-dihydroxy-(3alpha,5beta,12alpha)-; 3,12-Dihydroxycholan-24-oic acid, (3alpha,5beta,12alpha)-; 17-beta-(1-Methyl-3-carboxypropyl)-etiocholane-3-alpha,12-alpha-diol; l7-beta-(1-Methyl-3-carboxypropyl)-etiocholane-3-alpha,12-alpha-diol; 3.alpha.,12.alpha.-Dihydroxy-5.beta.-cholanic acid; 3.alpha.,12.alpha.-Dihydroxy-5.beta.-cholanoic acid; 3.alpha.,12.alpha.-Dihydroxy-5.beta.-cholan-24-oic acid; 5-beta-Cholan-24-oic acid, 3-alpha,12-alpha-dihydroxy-; Cholan-24-oic acid, 3,12-dihydroxy-, (3-alpha,5-beta,12-alpha)-; 17.beta.-(1-Methyl-3-carboxypropyl)-etiocholane-3.alpha.,12.alpha.-diol; ATX 101; DXC; SMR000112166; Deoxycholatate; de-oxycholate; deoxy-Cholate; UNII-005990WHZZ; Deoxycholic acid [USAN:INN]; deoxycholic-acid; ATX101; CCRIS 1627; HSDB 293; 5b-Deoxycholate; 3alpha; 7-Deoxycholate; deoxy-Cholic acid; EINECS 201-478-5; Kybella (TN); 5b-Deoxycholic acid; 7-Desoxycholic acid; 3-alpha,12-alpha-Dihydroxycholansaeure; BRN 3219882; 1e3v; Deoxycholic acid [INN]; Spectrum5_002007; bmse000833; SCHEMBL4300; Deoxycholic acid (NF/INN); BIDD:PXR0198; GTPL610; 4-10-00-01608 (Beilstein Handbook Reference); MLS001066423; MLS001306460; DEOXYCHOLIC ACID [II]; DEOXYCHOLIC ACID [MI]; CHEMBL406393; DEOXYCHOLIC ACID [HSDB]; DEOXYCHOLIC ACID [INCI]; DEOXYCHOLIC ACID [USAN]; DESOXYCHOLIC ACID [FCC]; 5b-Cholanic acid-3a,12a-diol; DTXSID0042662; DESOXYCHOLIC ACID [VANDF]; DEOXYCHOLIC ACID [USP-RS]; DEOXYCHOLIC ACID [WHO-DD]; HMS2270H22; HY-N0593; ZINC3914810; Deoxycholic acid, >=98% (HPLC); BBL013877; BDBM50375599; Deoxycholic acid, >=99.0% (T); LMST04010040; s4689; STK801948; AKOS005622617; DEOXYCHOLIC ACID [EP IMPURITY]; DEOXYCHOLIC ACID [ORANGE BOOK]; CCG-268565; CS-7613; DB03619; DEOXYCHOLIC ACID [USP MONOGRAPH]; AS-13233; BP-13275; NCI60_041946; 3-.alpha.,12-.alpha.-Dihydroxycholansaeure; Deoxycholic acid, BioXtra, >=98% (HPLC); C04483; D10781; D85117; EN300-108204; Q425680; SR-01000765601; 5.beta.-Cholan-24-oic acid,12.alpha.-dihydroxy-; SR-01000765601-2; 3I+/-,12I+/--Dihydroxy-5I(2)-cholansA currencyure; URSODEOXYCHOLIC ACID IMPURITY E [EP IMPURITY]; Z1416203075; 5.beta.-Cholan-24-oic acid, 3.alpha.,12.alpha.-dihydroxy-; 3,12-Dihydroxycholan-24-oic acid, (3.alpha.,5.beta.,12.alpha.)- #; 3alpha,12alpha-Dihydroxy-5beta-cholan-24-oic Acid (Deoxycholic Acid); Cholan-24-oic acid,12-dihydroxy-, (3.alpha.,5.beta.,12.alpha.)-; Deoxycholic acid, 500 mug/mL in methanol, certified reference material; (3alpha,5alpha,8alpha,12alpha,14beta,17alpha)-3,12-dihydroxycholan-24-oic acid; Cholan-24-oic acid, 3,12-dihydroxy-, (3-.alpha., 5-.beta.,12-.alpha.)-; Deoxycholic Acid (Desoxycholic Acid), United States Pharmacopeia (USP) Reference Standard; l7-.beta.-(1-Methyl-3-carboxypropyl)-etiocholane-3-.alpha.,12-.alpha.-diol; (4R)-4-((3R,8R,9S,10S,12S,13R,14S,17R)-3,12-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoic acid; (4R)-4-[(1R,3aS,3bR,5aR,7R,9aS,9bS,11S,11aR)-7,11-dihydroxy-9a,11a-dimethyl-hexadecahydro-1H-cyclopenta[a]phenanthren-1-yl]pentanoic acid
|
|
| CAS | 83-44-3 | |
| PubChem CID | 222528 | |
| ChEMBL ID | CHEMBL406393 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 392.6 | ALogp: | 4.9 |
| HBD: | 3 | HBA: | 4 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 77.8 | Aromatic Rings: | 4 |
| Heavy Atoms: | 28 | QED Weighted: | 0.631 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.147 | MDCK Permeability: | 0.00002200 |
| Pgp-inhibitor: | 0.982 | Pgp-substrate: | 0.987 |
| Human Intestinal Absorption (HIA): | 0.017 | 20% Bioavailability (F20%): | 0.039 |
| 30% Bioavailability (F30%): | 0.508 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.224 | Plasma Protein Binding (PPB): | 94.69% |
| Volume Distribution (VD): | 0.553 | Fu: | 3.42% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.032 | CYP1A2-substrate: | 0.203 |
| CYP2C19-inhibitor: | 0.009 | CYP2C19-substrate: | 0.773 |
| CYP2C9-inhibitor: | 0.032 | CYP2C9-substrate: | 0.914 |
| CYP2D6-inhibitor: | 0.002 | CYP2D6-substrate: | 0.156 |
| CYP3A4-inhibitor: | 0.027 | CYP3A4-substrate: | 0.087 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 10.596 | Half-life (T1/2): | 0.911 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.371 | Human Hepatotoxicity (H-HT): | 0.454 |
| Drug-inuced Liver Injury (DILI): | 0.04 | AMES Toxicity: | 0.004 |
| Rat Oral Acute Toxicity: | 0.609 | Maximum Recommended Daily Dose: | 0.871 |
| Skin Sensitization: | 0.939 | Carcinogencity: | 0.057 |
| Eye Corrosion: | 0.162 | Eye Irritation: | 0.505 |
| Respiratory Toxicity: | 0.918 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001007 | 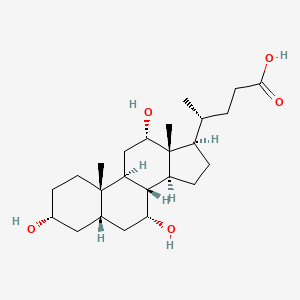 |
0.753 | D0OR2L | 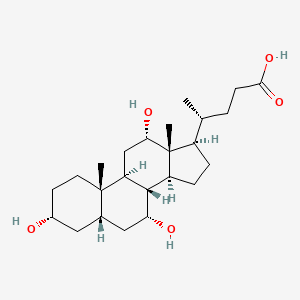 |
0.753 | ||
| ENC000609 | 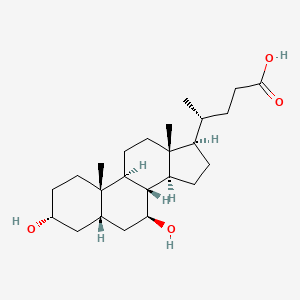 |
0.692 | D03ZTE | 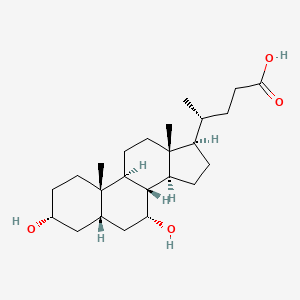 |
0.692 | ||
| ENC004228 | 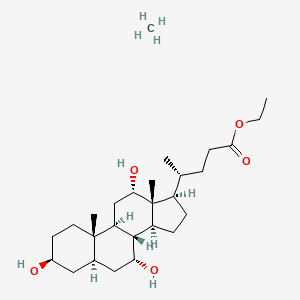 |
0.630 | D0G3SH | 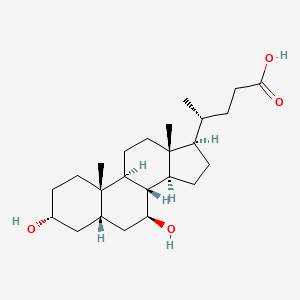 |
0.692 | ||
| ENC005147 | 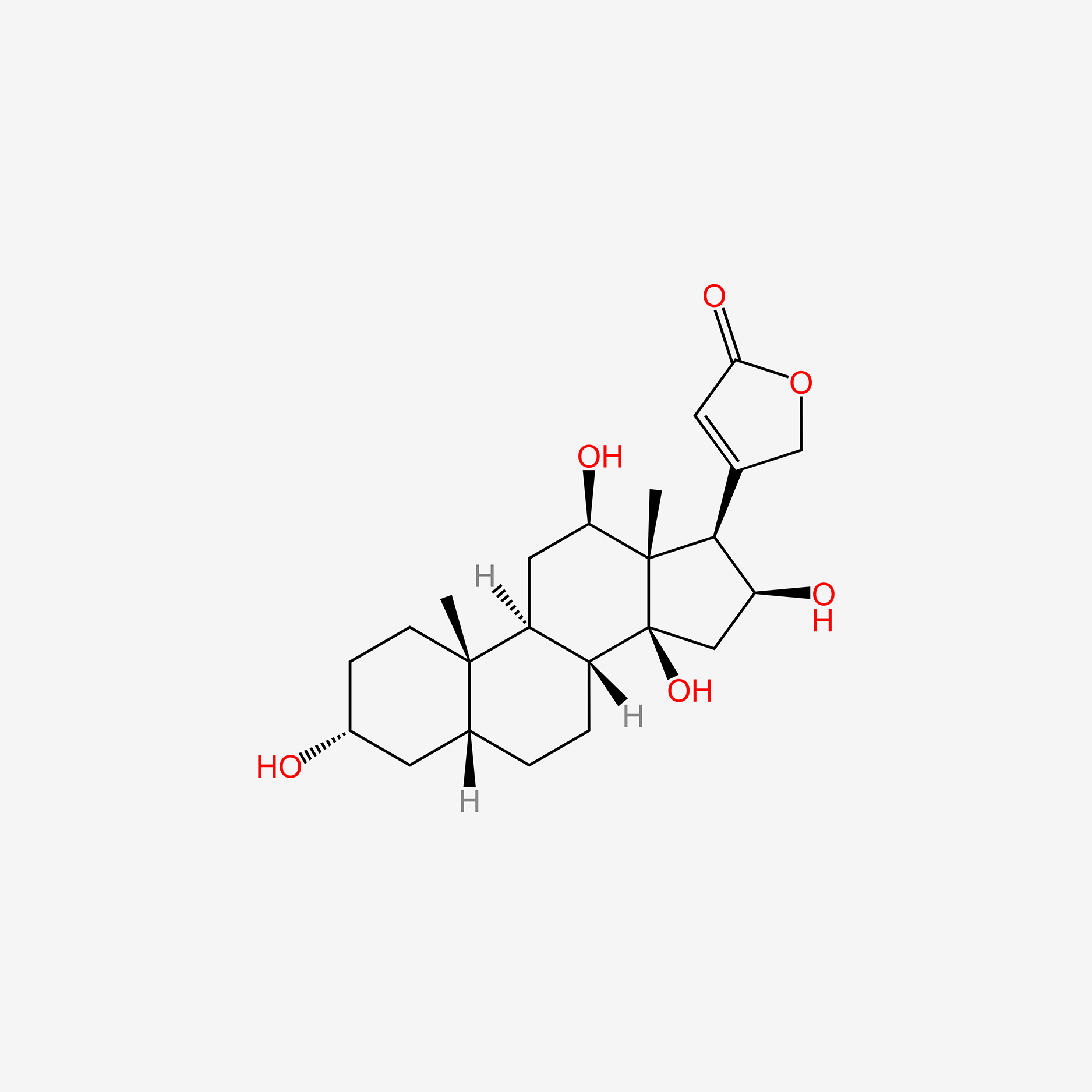 |
0.440 | D0M4WA | 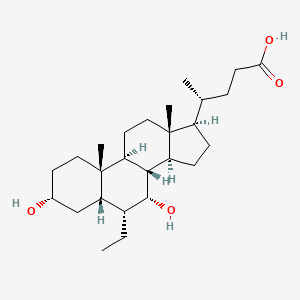 |
0.574 | ||
| ENC001603 | 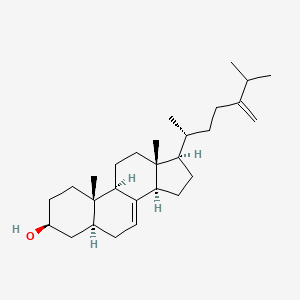 |
0.427 | D00VZZ | 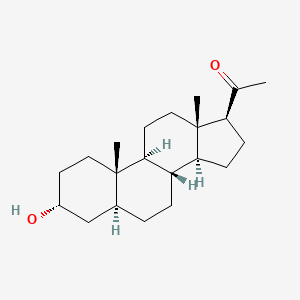 |
0.533 | ||
| ENC005968 | 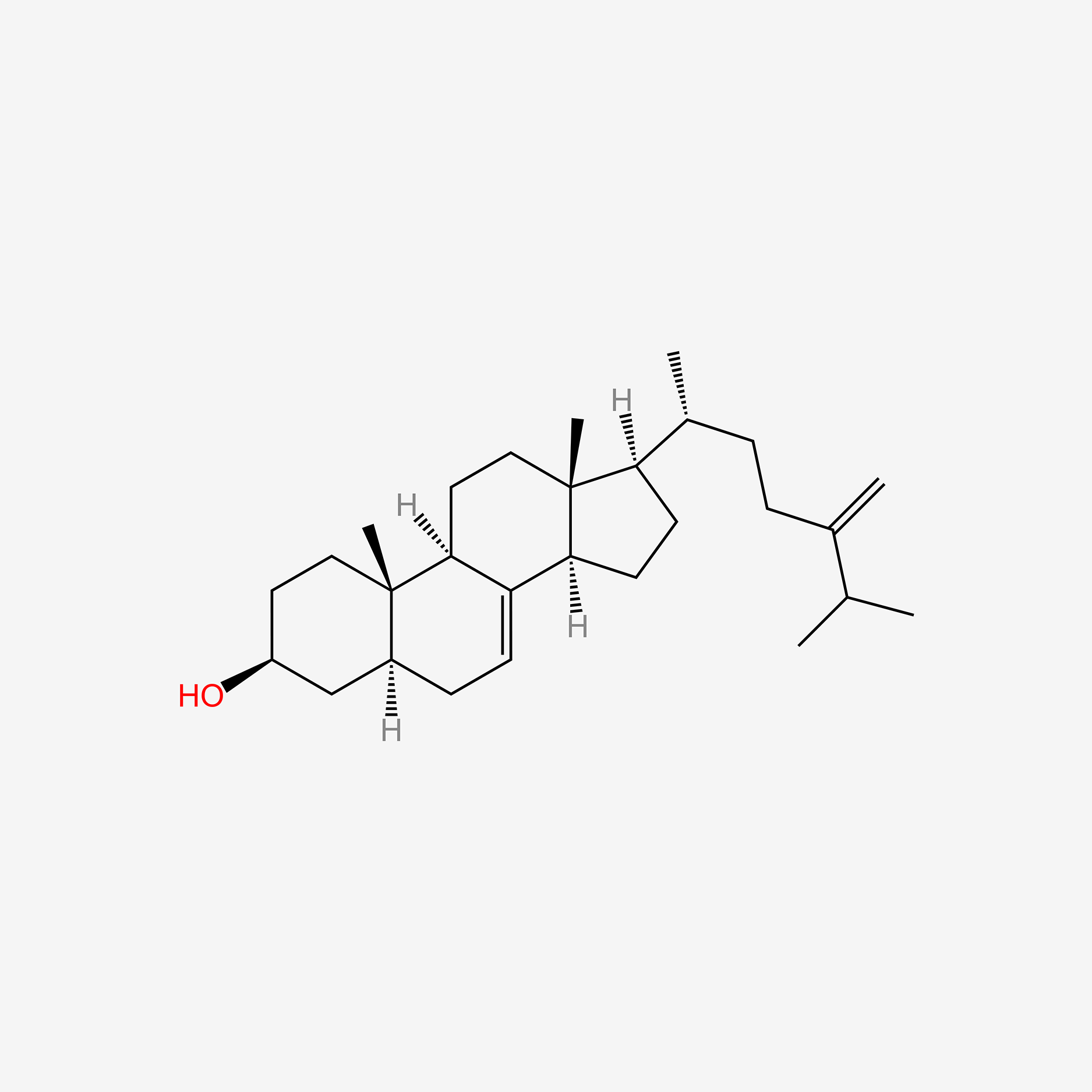 |
0.427 | D04DJN | 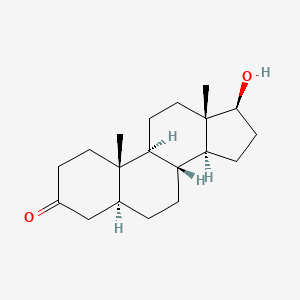 |
0.417 | ||
| ENC005144 | 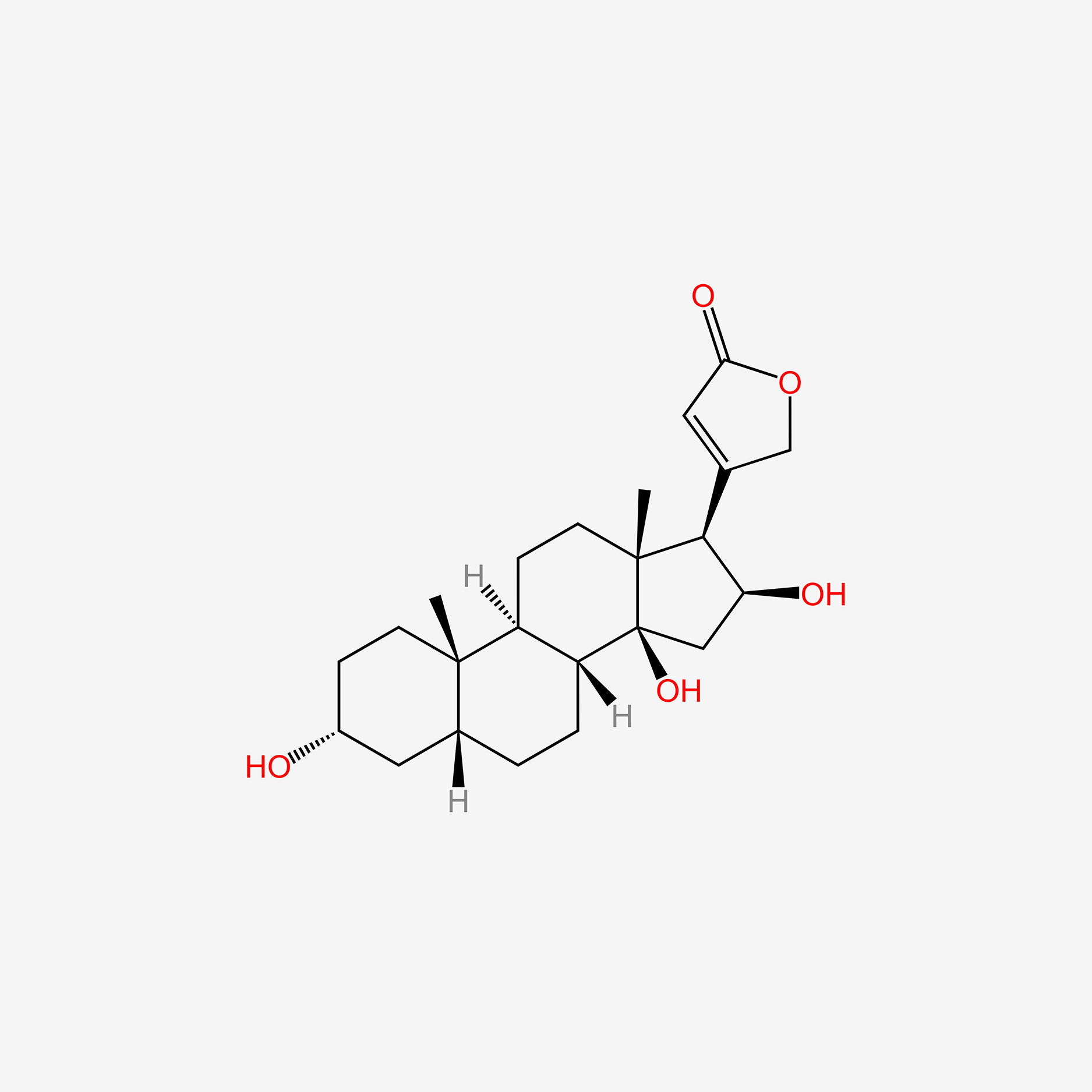 |
0.409 | D09NNA | 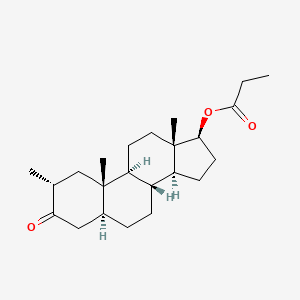 |
0.380 | ||
| ENC000125 | 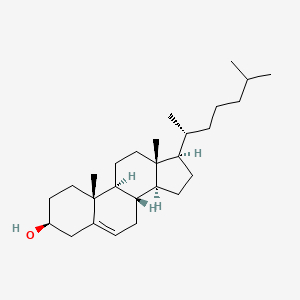 |
0.396 | D0Y7LD | 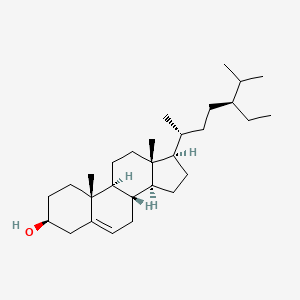 |
0.379 | ||
| ENC000961 | 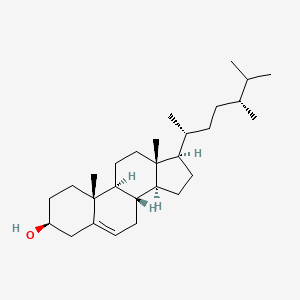 |
0.389 | D0B4RU | 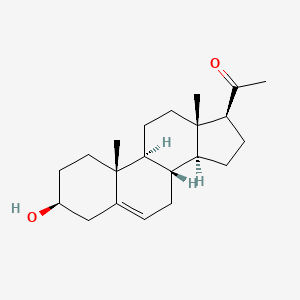 |
0.369 | ||
| ENC002216 | 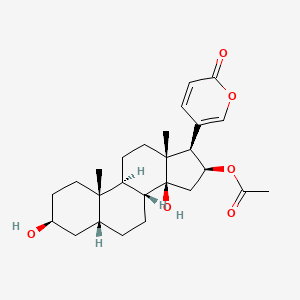 |
0.383 | D06XMU | 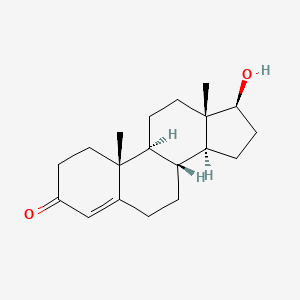 |
0.333 | ||