NPs Basic Information
|
Name |
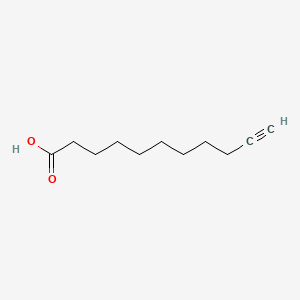
10-Undecynoic acid
|
| Molecular Formula | C11H18O2 | |
| IUPAC Name* |
undec-10-ynoic acid
|
|
| SMILES |
C#CCCCCCCCCC(=O)O
|
|
| InChI |
InChI=1S/C11H18O2/c1-2-3-4-5-6-7-8-9-10-11(12)13/h1H,3-10H2,(H,12,13)
|
|
| InChIKey |
OAOUTNMJEFWJPO-UHFFFAOYSA-N
|
|
| Synonyms |
10-Undecynoic acid; Undec-10-ynoic acid; 2777-65-3; Hendecynoic acid; 2F79G7H1WY; Undec-10-ynoicacid; EINECS 220-471-8; BRN 1704918; UNII-2F79G7H1WY; MFCD00014389; Undec-1-yn-11-oic acid; 10-HENDECYNOIC ACID; SCHEMBL40510; 10-Undecynoic acid, 95%; 4-02-00-01738 (Beilstein Handbook Reference); LCZC1163; DTXSID2075219; CHEBI:185054; ZINC2015667; GEO-02443; LMFA01030618; STL554898; AKOS001592014; AT18751; AS-57177; DB-047276; CS-0160163; FT-0607205; U0054; J-802018; W-200273; Q18611669
|
|
| CAS | 2777-65-3 | |
| PubChem CID | 31039 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 182.26 | ALogp: | 3.3 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 8 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 37.3 | Aromatic Rings: | 0 |
| Heavy Atoms: | 13 | QED Weighted: | 0.46 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.87 | MDCK Permeability: | 0.00002720 |
| Pgp-inhibitor: | 0.002 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.016 | 20% Bioavailability (F20%): | 0.172 |
| 30% Bioavailability (F30%): | 0.919 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.96 | Plasma Protein Binding (PPB): | 95.46% |
| Volume Distribution (VD): | 0.352 | Fu: | 1.81% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.09 | CYP1A2-substrate: | 0.411 |
| CYP2C19-inhibitor: | 0.042 | CYP2C19-substrate: | 0.4 |
| CYP2C9-inhibitor: | 0.143 | CYP2C9-substrate: | 0.966 |
| CYP2D6-inhibitor: | 0.025 | CYP2D6-substrate: | 0.132 |
| CYP3A4-inhibitor: | 0.023 | CYP3A4-substrate: | 0.034 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 1.771 | Half-life (T1/2): | 0.808 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.017 | Human Hepatotoxicity (H-HT): | 0.067 |
| Drug-inuced Liver Injury (DILI): | 0.039 | AMES Toxicity: | 0.014 |
| Rat Oral Acute Toxicity: | 0.164 | Maximum Recommended Daily Dose: | 0.044 |
| Skin Sensitization: | 0.686 | Carcinogencity: | 0.552 |
| Eye Corrosion: | 0.955 | Eye Irritation: | 0.987 |
| Respiratory Toxicity: | 0.761 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000088 |  |
0.683 | D0Z5BC |  |
0.636 | ||
| ENC000270 |  |
0.636 | D0E4WR |  |
0.543 | ||
| ENC000720 |  |
0.634 | D0FD0H |  |
0.463 | ||
| ENC000263 |  |
0.610 | D0Y8DP |  |
0.400 | ||
| ENC000102 | 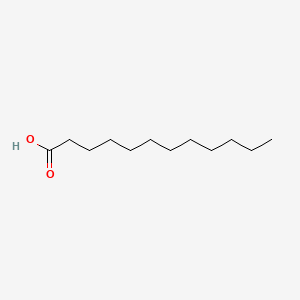 |
0.596 | D0XN8C |  |
0.397 | ||
| ENC001228 | 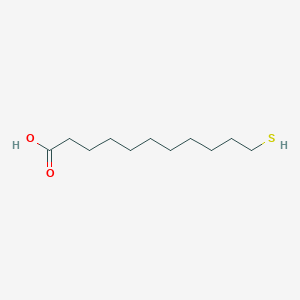 |
0.596 | D0O1PH |  |
0.378 | ||
| ENC000647 | 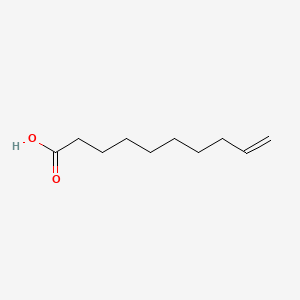 |
0.568 | D0O1TC | 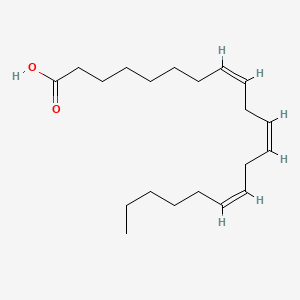 |
0.356 | ||
| ENC000075 |  |
0.543 | D07ILQ |  |
0.333 | ||
| ENC000030 |  |
0.537 | D0I4DQ |  |
0.329 | ||
| ENC000378 |  |
0.528 | D03ZJE |  |
0.319 | ||