NPs Basic Information
|
Name |
Myristic Acid
|
| Molecular Formula | C14H28O2 | |
| IUPAC Name* |
tetradecanoic acid
|
|
| SMILES |
CCCCCCCCCCCCCC(=O)O
|
|
| InChI |
InChI=1S/C14H28O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14(15)16/h2-13H2,1H3,(H,15,16)
|
|
| InChIKey |
TUNFSRHWOTWDNC-UHFFFAOYSA-N
|
|
| Synonyms |
Tetradecanoic acid; MYRISTIC ACID; 544-63-8; n-Tetradecanoic acid; n-Tetradecan-1-oic acid; Crodacid; n-Tetradecoic acid; 1-Tridecanecarboxylic acid; Myristate; tetradecoic acid; Hydrofol acid 1495; Univol U 316S; Emery 655; Myristinsaeure; Hystrene 9014; FEMA No. 2764; Myristic acid, pure; n-Myristic acid; Myristic acid (natural); Tetradecanoate; acide tetradecanoique; NSC 5028; CCRIS 4724; CH3-[CH2]12-COOH; HSDB 5686; C14:0; Philacid 1400; CHEBI:28875; AI3-15381; Prifac 2942; NSC-5028; 1-tetradecanecarboxylic acid; PHILACID-1400; PRIFRAC-2942; 0I3V7S25AW; CHEMBL111077; NSC5028; DSSTox_CID_1666; n-tetradecan-1-oate; DSSTox_RID_76274; DSSTox_GSID_21666; 32112-52-0; CAS-544-63-8; Myristic acid [NF]; EINECS 208-875-2; BRN 0508624; myristoate; UNII-0I3V7S25AW; myristoic acid; n-Tetradecanoate; Tetradecanoicacid; 3usx; Myristic acid pure; Myristic Acid Flake; MFCD00002744; Hystrene 9514; Edenor C 14; Myristic Acid 655; 1-Tridecanecarboxylate; MAGNESIUMARSENATE; Myristic acid, 95%; Myristic acid, natural; tridecanecarboxylic acid; Myristic acid (8CI); Myristic Acid, Reagent; 3v2n; 3w9k; Myristic acid, puriss.; Tetradecanoic acid (9CI); bmse000737; Epitope ID:176772; MYRISTIC ACID [II]; MYRISTIC ACID [MI]; SCHEMBL6374; MYRISTIC ACID [FCC]; Myristic acid-[14-13C]; MYRISTIC ACID [FHFI]; MYRISTIC ACID [HSDB]; MYRISTIC ACID [INCI]; 4-02-00-01126 (Beilstein Handbook Reference); MLS002152942; WLN: QV13; Tetradecanoic (Myristic) acid; MYRISTIC ACID [MART.]; GTPL2806; MYRISTIC ACID [USP-RS]; DTXSID6021666; HMS3039E15; HMS3648O20; Myristic acid, analytical standard; HY-N2041; ZINC1530417; EINECS 250-924-5; Myristic acid, >=98.0% (GC); Tox21_201852; Tox21_302781; BDBM50147581; LMFA01010014; s5617; STL185697; Myristic acid, >=95%, FCC, FG; Myristic acid, Sigma Grade, >=99%; AKOS009156714; CCG-266785; DB08231; DS-3833; FA 14:0; NSC 122834; NCGC00091068-01; NCGC00091068-02; NCGC00091068-03; NCGC00256547-01; NCGC00259401-01; AC-34674; BP-27915; SMR001224536; CS-0018531; FT-0602832; FT-0770860; M0476; EN300-78099; C06424; Myristic acid, Vetec(TM) reagent grade, 98%; Q422658; SR-01000854525; SR-01000854525-3; W-109088; F8889-5016; Z1954802504; EDAE4876-C383-4AD4-A419-10C0550931DB; MYRISTIC ACID (CONSTITUENT OF SAW PALMETTO) [DSC]; Myristic acid, United States Pharmacopeia (USP) Reference Standard; Tetradecanoic acid; 1-Tridecanecarboxylic acid; n-Tetradecanoic acid; Myristic acid, Pharmaceutical Secondary Standard; Certified Reference Material
|
|
| CAS | 544-63-8 | |
| PubChem CID | 11005 | |
| ChEMBL ID | CHEMBL111077 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 228.37 | ALogp: | 5.3 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 12 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 37.3 | Aromatic Rings: | 0 |
| Heavy Atoms: | 16 | QED Weighted: | 0.467 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.96 | MDCK Permeability: | 0.00002780 |
| Pgp-inhibitor: | 0.028 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.754 |
| 30% Bioavailability (F30%): | 0.987 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.163 | Plasma Protein Binding (PPB): | 98.20% |
| Volume Distribution (VD): | 0.416 | Fu: | 1.28% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.256 | CYP1A2-substrate: | 0.202 |
| CYP2C19-inhibitor: | 0.099 | CYP2C19-substrate: | 0.213 |
| CYP2C9-inhibitor: | 0.249 | CYP2C9-substrate: | 0.986 |
| CYP2D6-inhibitor: | 0.007 | CYP2D6-substrate: | 0.069 |
| CYP3A4-inhibitor: | 0.017 | CYP3A4-substrate: | 0.024 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 2.303 | Half-life (T1/2): | 0.718 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.04 | Human Hepatotoxicity (H-HT): | 0.03 |
| Drug-inuced Liver Injury (DILI): | 0.042 | AMES Toxicity: | 0.006 |
| Rat Oral Acute Toxicity: | 0.037 | Maximum Recommended Daily Dose: | 0.015 |
| Skin Sensitization: | 0.807 | Carcinogencity: | 0.078 |
| Eye Corrosion: | 0.979 | Eye Irritation: | 0.983 |
| Respiratory Toxicity: | 0.848 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
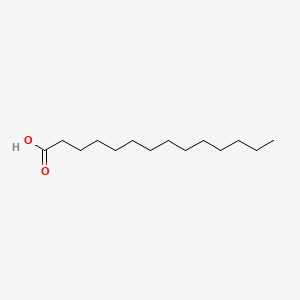
| ENC000466 | 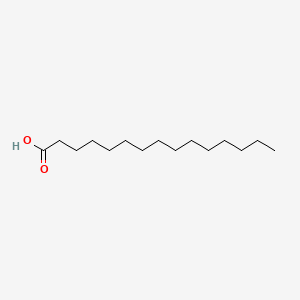 |
0.938 | D07ILQ |  |
0.641 | ||
| ENC000050 | 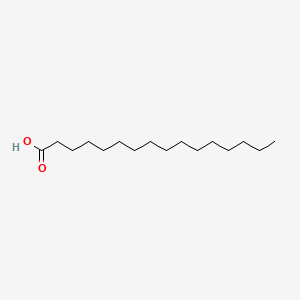 |
0.882 | D0O1PH |  |
0.632 | ||
| ENC000102 |  |
0.867 | D0Z5SM |  |
0.571 | ||
| ENC000356 | 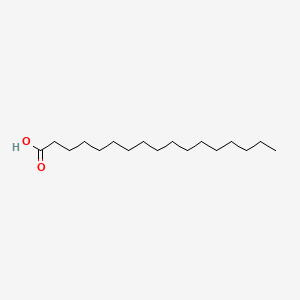 |
0.833 | D05ATI |  |
0.559 | ||
| ENC000270 | 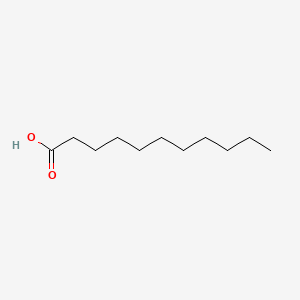 |
0.800 | D0XN8C |  |
0.529 | ||
| ENC000642 | 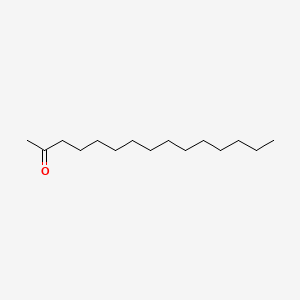 |
0.800 | D0Z5BC |  |
0.528 | ||
| ENC000110 | 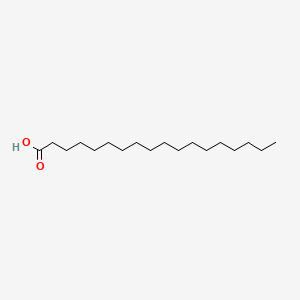 |
0.789 | D0O1TC |  |
0.479 | ||
| ENC000604 |  |
0.755 | D00AOJ |  |
0.474 | ||
| ENC000088 |  |
0.733 | D0P1RL |  |
0.468 | ||
| ENC001100 | 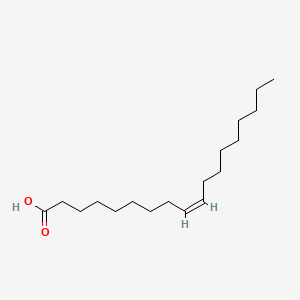 |
0.729 | D0E4WR | 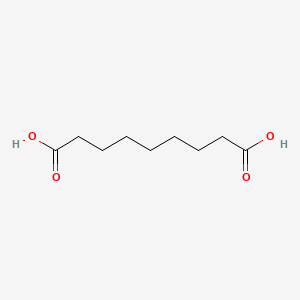 |
0.455 | ||