NPs Basic Information
|
Name |
1-Tridecene
|
| Molecular Formula | C13H26 | |
| IUPAC Name* |
tridec-1-ene
|
|
| SMILES |
CCCCCCCCCCCC=C
|
|
| InChI |
InChI=1S/C13H26/c1-3-5-7-9-11-13-12-10-8-6-4-2/h3H,1,4-13H2,2H3
|
|
| InChIKey |
VQOXUMQBYILCKR-UHFFFAOYSA-N
|
|
| Synonyms |
1-TRIDECENE; 2437-56-1; tridec-1-ene; N-Tridec-1-ene; Tridecylene; Undecylethylene; alpha-Tridecene; Tridecene-1; .alpha.-Tridecene; 1-Tridecene, 97%; CHEBI:89815; 5B0U2S314C; NSC-78473; 1-Tridecene [Standard Material for GC]; 1-C13H26; CCRIS 5719; HSDB 1088; EINECS 219-443-8; NSC 78473; BRN 1744660; UNII-5B0U2S314C; 1-Tridecylene; MFCD00008976; 1-Tridecene, 96%; DSSTox_CID_9235; DSSTox_RID_78723; 1-TRIDECENE [HSDB]; DSSTox_GSID_29235; 4-01-00-00921 (Beilstein Handbook Reference); CHEMBL3186542; DTXSID3029235; CAA43756; NSC78473; ZINC1718855; Tox21_202997; LMFA11000331; AKOS015903313; NCGC00260542-01; BP-13303; LS-14252; CAS-2437-56-1; DB-046427; CS-0186370; FT-0608321; T0637; D92374; J-015475; Q27162003; N-(2-AMINOETHYL)-N-METHYL-N-PHENYLAMINEDIHYDROCHLORIDE
|
|
| CAS | 2437-56-1 | |
| PubChem CID | 17095 | |
| ChEMBL ID | CHEMBL3186542 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 182.35 | ALogp: | 7.3 |
| HBD: | 0 | HBA: | 0 |
| Rotatable Bonds: | 10 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 0.0 | Aromatic Rings: | 0 |
| Heavy Atoms: | 13 | QED Weighted: | 0.313 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.602 | MDCK Permeability: | 0.00001410 |
| Pgp-inhibitor: | 0.02 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.269 |
| 30% Bioavailability (F30%): | 0.668 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.884 | Plasma Protein Binding (PPB): | 98.52% |
| Volume Distribution (VD): | 1.819 | Fu: | 1.96% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.836 | CYP1A2-substrate: | 0.23 |
| CYP2C19-inhibitor: | 0.58 | CYP2C19-substrate: | 0.175 |
| CYP2C9-inhibitor: | 0.292 | CYP2C9-substrate: | 0.924 |
| CYP2D6-inhibitor: | 0.18 | CYP2D6-substrate: | 0.462 |
| CYP3A4-inhibitor: | 0.546 | CYP3A4-substrate: | 0.093 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 4.705 | Half-life (T1/2): | 0.145 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.067 | Human Hepatotoxicity (H-HT): | 0.011 |
| Drug-inuced Liver Injury (DILI): | 0.04 | AMES Toxicity: | 0.012 |
| Rat Oral Acute Toxicity: | 0.034 | Maximum Recommended Daily Dose: | 0.032 |
| Skin Sensitization: | 0.955 | Carcinogencity: | 0.086 |
| Eye Corrosion: | 0.994 | Eye Irritation: | 0.979 |
| Respiratory Toxicity: | 0.369 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000475 |  |
0.925 | D0Z5BC |  |
0.553 | ||
| ENC000273 |  |
0.919 | D05ATI |  |
0.527 | ||
| ENC000573 |  |
0.860 | D0Z5SM |  |
0.468 | ||
| ENC000455 | 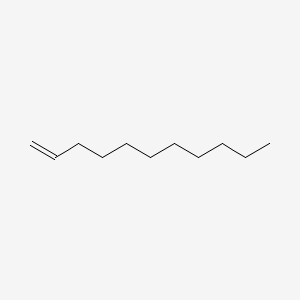 |
0.838 | D0O1PH |  |
0.451 | ||
| ENC000425 | 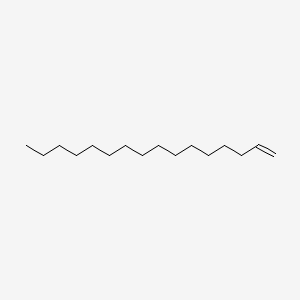 |
0.804 | D07ILQ |  |
0.426 | ||
| ENC000277 | 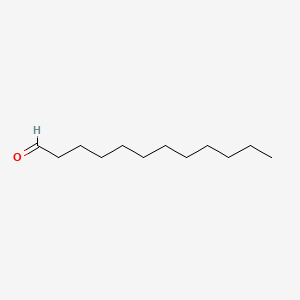 |
0.762 | D05QNO | 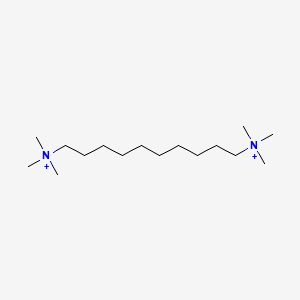 |
0.417 | ||
| ENC000460 |  |
0.757 | D0Y8DP |  |
0.393 | ||
| ENC000557 |  |
0.755 | D00AOJ |  |
0.387 | ||
| ENC000283 |  |
0.712 | D0XN8C |  |
0.352 | ||
| ENC001644 | 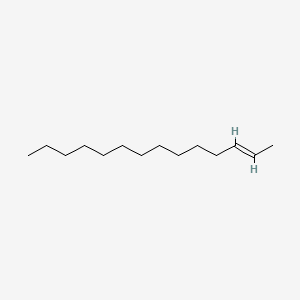 |
0.711 | D0O1TC | 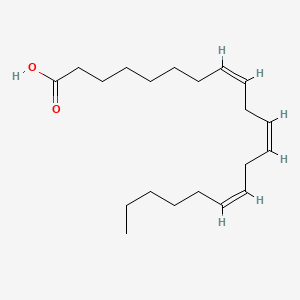 |
0.351 | ||