NPs Basic Information
|
Name |
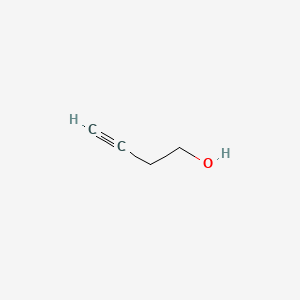
3-Butyn-1-OL
|
| Molecular Formula | C4H6O | |
| IUPAC Name* |
but-3-yn-1-ol
|
|
| SMILES |
C#CCCO
|
|
| InChI |
InChI=1S/C4H6O/c1-2-3-4-5/h1,5H,3-4H2
|
|
| InChIKey |
OTJZCIYGRUNXTP-UHFFFAOYSA-N
|
|
| Synonyms |
3-BUTYN-1-OL; But-3-yn-1-ol; 927-74-2; 3-Butynol; 3-Butyne-1-ol; 4-Hydroxy-1-butyne; 3-Butynyl alcohol; 1-Butyn-4-ol; 2-Hydroxyethylacetylene; (2-Hydroxyethyl)acetylene; homopropargyl alcohol; 4-hydroxy-butyne; NSC 9708; 4-butyne-1-ol; 4-hydroxy-but-1-yne; NSC-9708; P74L430293; 1-butyne-4-ol; EINECS 213-161-9; MFCD00002955; BRN 0773710; 4-hydroxybutyne; AI3-25453; 3-butyn-ol; UNII-P74L430293; 3butyn-1-ol; 1-hydroxy-3-butyne; 1-hydroxybut-3-yne; 3-butin-1-ol; 3-butyn-1 ol; but-3-yne-1-ol; homopropargylic alcohol; 3-but-yn-1-ol; bmse000362; HC.$.CCH2CH2OH; WLN: Q3UU1; 3-Butyn-1-ol, 97%; 4-01-00-02219 (Beilstein Handbook Reference); But-3-yn-1-ol;3-Butynol; DTXSID1022136; CHEBI:27444; NSC9708; ACT08938; BCP33461; STR09821; ZINC1700120; AKOS000121102; CS-W001947; SB40659; BP-31076; DB-028414; B0799; FT-0615274; EN300-34598; C06146; A844341; Q223060; F0001-2231
|
|
| CAS | 927-74-2 | |
| PubChem CID | 13566 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 70.09 | ALogp: | 0.1 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 20.2 | Aromatic Rings: | 0 |
| Heavy Atoms: | 5 | QED Weighted: | 0.443 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.143 | MDCK Permeability: | 0.00003940 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.006 | 20% Bioavailability (F20%): | 0.395 |
| 30% Bioavailability (F30%): | 0.808 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.998 | Plasma Protein Binding (PPB): | 42.45% |
| Volume Distribution (VD): | 0.892 | Fu: | 60.29% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.283 | CYP1A2-substrate: | 0.779 |
| CYP2C19-inhibitor: | 0.034 | CYP2C19-substrate: | 0.632 |
| CYP2C9-inhibitor: | 0.006 | CYP2C9-substrate: | 0.581 |
| CYP2D6-inhibitor: | 0.015 | CYP2D6-substrate: | 0.167 |
| CYP3A4-inhibitor: | 0.009 | CYP3A4-substrate: | 0.176 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 6.338 | Half-life (T1/2): | 0.877 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.012 | Human Hepatotoxicity (H-HT): | 0.224 |
| Drug-inuced Liver Injury (DILI): | 0.035 | AMES Toxicity: | 0.265 |
| Rat Oral Acute Toxicity: | 0.278 | Maximum Recommended Daily Dose: | 0.076 |
| Skin Sensitization: | 0.634 | Carcinogencity: | 0.433 |
| Eye Corrosion: | 0.942 | Eye Irritation: | 0.996 |
| Respiratory Toxicity: | 0.696 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000508 | 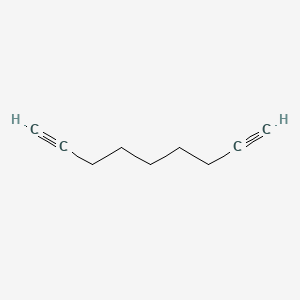 |
0.267 | D0C1QZ | 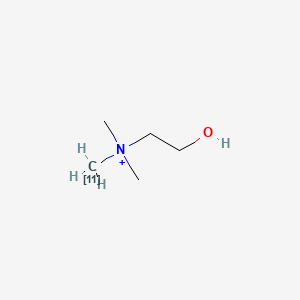 |
0.200 | ||
| ENC000017 | 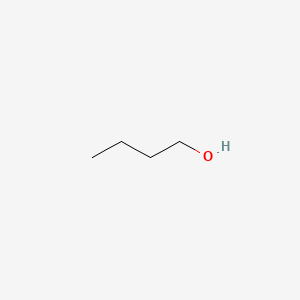 |
0.238 | D0EP8X | 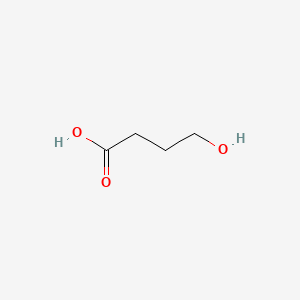 |
0.192 | ||
| ENC000686 | 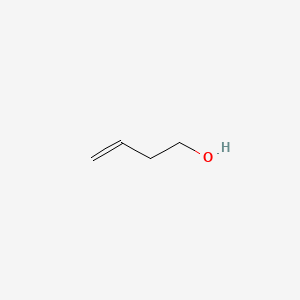 |
0.238 | D00AMQ | 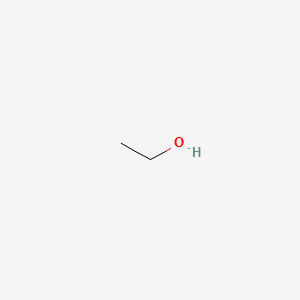 |
0.188 | ||
| ENC000593 | 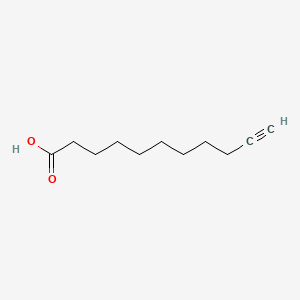 |
0.225 | D0X2IE | 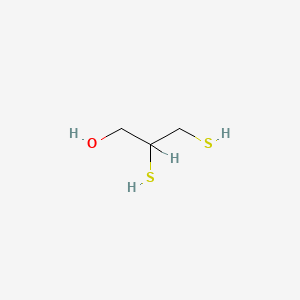 |
0.167 | ||
| ENC000720 | 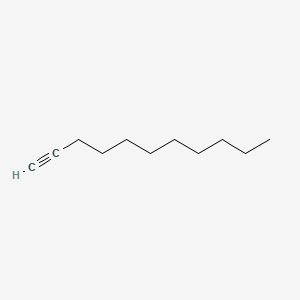 |
0.222 | D09KDV | 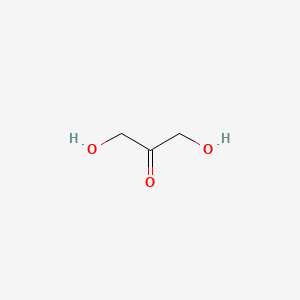 |
0.167 | ||
| ENC000453 | 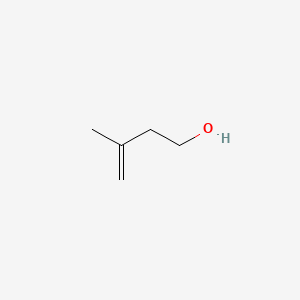 |
0.217 | D03VZH | 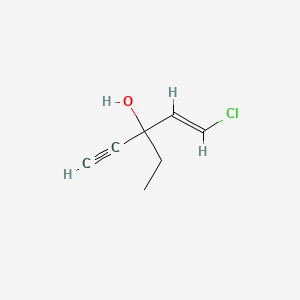 |
0.161 | ||
| ENC000677 | 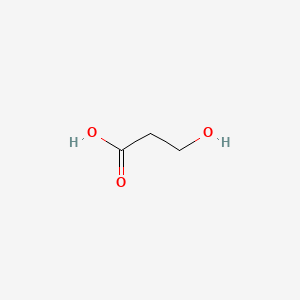 |
0.217 | D0R0UJ | 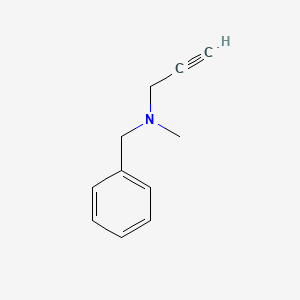 |
0.146 | ||
| ENC000600 | 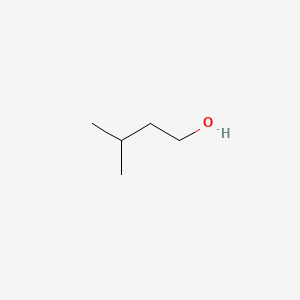 |
0.217 | D03ZFN | 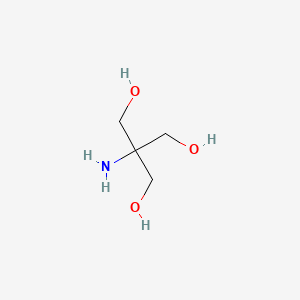 |
0.138 | ||
| ENC000355 | 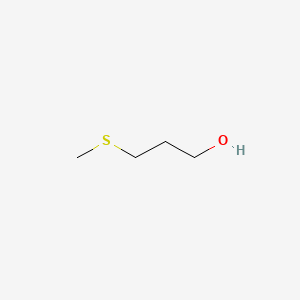 |
0.208 | D03CHT | 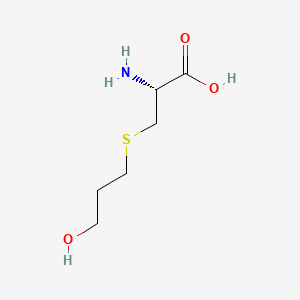 |
0.135 | ||
| ENC000139 | 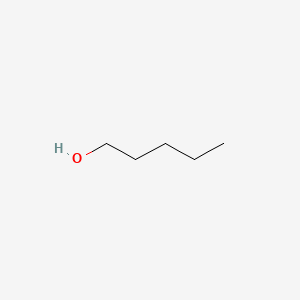 |
0.208 | D0S2UG | 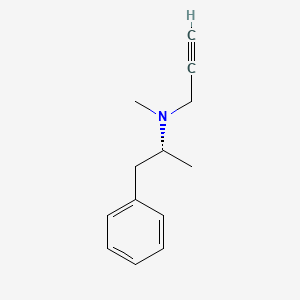 |
0.130 | ||