NPs Basic Information
|
Name |
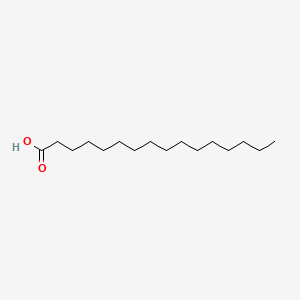
Palmitic Acid
|
| Molecular Formula | C16H32O2 | |
| IUPAC Name* |
hexadecanoic acid
|
|
| SMILES |
CCCCCCCCCCCCCCCC(=O)O
|
|
| InChI |
InChI=1S/C16H32O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16(17)18/h2-15H2,1H3,(H,17,18)
|
|
| InChIKey |
IPCSVZSSVZVIGE-UHFFFAOYSA-N
|
|
| Synonyms |
palmitic acid; Hexadecanoic acid; 57-10-3; Cetylic acid; palmitate; n-Hexadecanoic acid; Hexadecylic acid; Hydrofol; n-Hexadecoic acid; 1-Pentadecanecarboxylic acid; Palmitinic acid; Pentadecanecarboxylic acid; hexadecanoate; hexaectylic acid; 1-Hexyldecanoic Acid; hexadecoic acid; Industrene 4516; Emersol 140; Emersol 143; Hystrene 8016; Hystrene 9016; Palmitinsaeure; Palmitic acid, pure; FEMA No. 2832; Palmitic acid 95%; Kortacid 1698; Loxiol EP 278; Palmitic acid (natural); Hydrofol Acid 1690; Prifac 2960; Pristerene 4934; Edenor C16; Lunac P 95KC; C16:0; Lunac P 95; Lunac P 98; Cetyl acid; HSDB 5001; AI3-01594; NSC 5030; Pristerene-4934; Palmitic acid (NF); Glycon P-45; CHEBI:15756; NSC5030; Prifac-2960; NSC-5030; Hexadecanoic acid (9CI); MFCD00002747; Palmitic acid (7CI,8CI); CHEMBL82293; CH3-[CH2]14-COOH; IMEX C 1498; 2V16EO95H1; n-hexadecoate; LMFA01010001; PA 900; 67701-02-4; FA 16:0; FA 1695; 1-hexyldecanoate; NCGC00164358-01; DSSTox_CID_1602; pentadecanecarboxylate; Hexadecanoic acid 10 microg/mL in Acetonitrile; DSSTox_RID_76229; DSSTox_GSID_21602; C16H32O2; PLM; palmic acid; Hexadecanoate (n-C16:0); CAS-57-10-3; CCRIS 5443; SR-01000944716; EINECS 200-312-9; Palmitic acid [USAN:NF]; BRN 0607489; palmitoate; Hexadecoate; Palmitinate; palmitic-acid; palmitoic acid; Hexadecanoicacid; Aethalic acid; UNII-2V16EO95H1; Hexadecanoic acid Palmitic acid; 2hmb; 2hnx; Palmitic acid_jeyam; Palmitic Acid, FCC; Kortacid 1695; Palmitic acid_RaGuSa; Univol U332; Prifrac 2960; Hexadecanoic acid anion; 3v2q; Palmitic acid, >=99%; bmse000590; Epitope ID:141181; EC 200-312-9; CETYL ACID [VANDF]; PALMITIC ACID [II]; PALMITIC ACID [MI]; SCHEMBL6177; PALMITIC ACID [DSC]; PALMITIC ACID [FCC]; PALMITIC ACID [FHFI]; PALMITIC ACID [HSDB]; PALMITIC ACID [INCI]; PALMITIC ACID [USAN]; 4-02-00-01157 (Beilstein Handbook Reference); FAT; WLN: QV15; P5585_SIGMA; PALMITIC ACID [VANDF]; PALMITIC ACID [MART.]; GTPL1055; QSPL 166; PALMITIC ACID [USP-RS]; PALMITIC ACID [WHO-DD]; (1(1)(3)C)hexadecanoic acid; DTXSID2021602; 1b56; HMS3649N08; Palmitic acid, analytical standard; Palmitic acid, BioXtra, >=99%; Palmitic acid, Grade II, ~95%; HY-N0830; Palmitic acid, natural, 98%, FG; ZINC6072466; Tox21_112105; Tox21_201671; Tox21_302966; AC9381; BBL011563; BDBM50152850; PALMITIC ACID [EP MONOGRAPH]; s3794; STL146733; EDENOR C 16-98-100; Palmitic acid, >=95%, FCC, FG; AKOS005720983; Tox21_112105_1; CCG-267027; CR-0047; DB03796; Palmitic acid, for synthesis, 98.0%; SURFAXIN COMPONENT PALMITIC ACID; NCGC00164358-02; NCGC00164358-03; NCGC00256424-01; NCGC00259220-01; BP-27917; LUCINACTANT COMPONENT PALMITIC ACID; Palmitic acid, purum, >=98.0% (GC); SY006518; CS-0009861; FT-0626965; FT-0772579; P0002; P1145; Palmitic acid, SAJ first grade, >=95.0%; EN300-19603; A14813; C00249; D05341; Palmitic acid, Vetec(TM) reagent grade, 98%; Palmitic acid, >=98% palmitic acid basis (GC); A831313; HEXADECANOIC ACID-13C16 (ALGAL SOURCE) (; Q209727; SR-01000944716-1; SR-01000944716-2; BA71C79B-C9B1-451A-A5BE-B480B5CC7D0C; PALMITIC ACID (CONSTITUENT OF SPIRULINA) [DSC]; F0001-1488; Z104474418; PALMITIC ACID (CONSTITUENT OF SAW PALMETTO) [DSC]; Palmitic acid, certified reference material, TraceCERT(R); Palmitic acid, European Pharmacopoeia (EP) Reference Standard; Palmitic acid, United States Pharmacopeia (USP) Reference Standard; Palmitic acid, Pharmaceutical Secondary Standard; Certified Reference Material; Sodium Palmitate, Palmitic acid sodium salt, Sodium hexadecanoate, Sodium pentadecanecarboxylate, HSDB 759
|
|
| CAS | 57-10-3 | |
| PubChem CID | 985 | |
| ChEMBL ID | CHEMBL82293 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 256.42 | ALogp: | 6.4 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 14 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 37.3 | Aromatic Rings: | 0 |
| Heavy Atoms: | 18 | QED Weighted: | 0.403 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.027 | MDCK Permeability: | 0.00002470 |
| Pgp-inhibitor: | 0.009 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.644 |
| 30% Bioavailability (F30%): | 0.991 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.06 | Plasma Protein Binding (PPB): | 98.95% |
| Volume Distribution (VD): | 0.608 | Fu: | 1.01% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.3 | CYP1A2-substrate: | 0.194 |
| CYP2C19-inhibitor: | 0.203 | CYP2C19-substrate: | 0.11 |
| CYP2C9-inhibitor: | 0.174 | CYP2C9-substrate: | 0.989 |
| CYP2D6-inhibitor: | 0.008 | CYP2D6-substrate: | 0.054 |
| CYP3A4-inhibitor: | 0.024 | CYP3A4-substrate: | 0.019 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 2.377 | Half-life (T1/2): | 0.61 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.056 | Human Hepatotoxicity (H-HT): | 0.026 |
| Drug-inuced Liver Injury (DILI): | 0.043 | AMES Toxicity: | 0.005 |
| Rat Oral Acute Toxicity: | 0.029 | Maximum Recommended Daily Dose: | 0.015 |
| Skin Sensitization: | 0.899 | Carcinogencity: | 0.064 |
| Eye Corrosion: | 0.977 | Eye Irritation: | 0.978 |
| Respiratory Toxicity: | 0.891 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000356 | 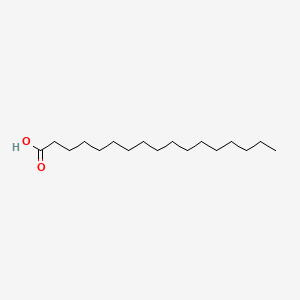 |
0.944 | D07ILQ |  |
0.734 | ||
| ENC000466 | 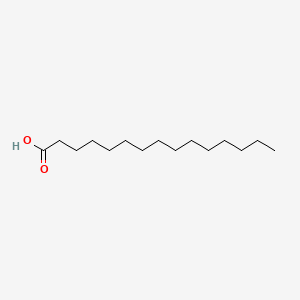 |
0.941 | D0O1PH |  |
0.625 | ||
| ENC000110 | 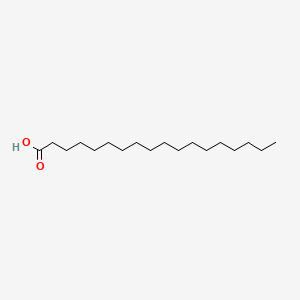 |
0.895 | D0Z5SM |  |
0.591 | ||
| ENC000378 |  |
0.882 | D00AOJ |  |
0.553 | ||
| ENC000688 |  |
0.821 | D05ATI |  |
0.508 | ||
| ENC000357 |  |
0.810 | D00FGR |  |
0.500 | ||
| ENC000271 | 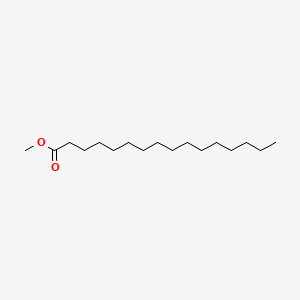 |
0.780 | D0XN8C |  |
0.486 | ||
| ENC000102 | 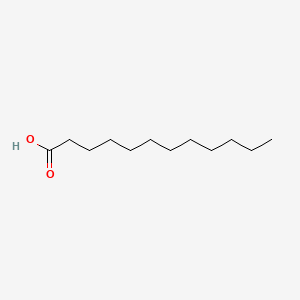 |
0.765 | D0Z5BC |  |
0.475 | ||
| ENC000426 | 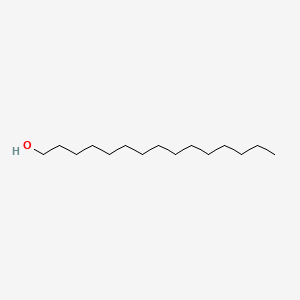 |
0.764 | D0P1RL | 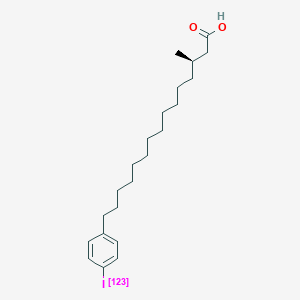 |
0.452 | ||
| ENC001593 |  |
0.754 | D0O1TC |  |
0.443 | ||