NPs Basic Information
|
Name |
Physcion
|
| Molecular Formula | C16H12O5 | |
| IUPAC Name* |
1,8-dihydroxy-3-methoxy-6-methylanthracene-9,10-dione
|
|
| SMILES |
CC1=CC2=C(C(=C1)O)C(=O)C3=C(C2=O)C=C(C=C3O)OC
|
|
| InChI |
InChI=1S/C16H12O5/c1-7-3-9-13(11(17)4-7)16(20)14-10(15(9)19)5-8(21-2)6-12(14)18/h3-6,17-18H,1-2H3
|
|
| InChIKey |
FFWOKTFYGVYKIR-UHFFFAOYSA-N
|
|
| Synonyms |
Physcion; 521-61-9; Physcione; Parietin; Rheochrysidin; Emodin 3-methyl ether; 1,8-Dihydroxy-3-methoxy-6-methylanthraquinone; 1,8-dihydroxy-3-methoxy-6-methylanthracene-9,10-dione; Emodin monomethyl ether; Emodin-3-methyl ether; NSC 251670; 1,8-Dihydroxy-3-methoxy-6-methyl-9,10-anthracenedione; Parienin; 1,8-Dihydroxy-3-methyl-6-methoxyanthraquinone; Parietin;Rheochrysidin; Rheochrysidin (Physcione); NSC251670; 1,8-Dihydroxy-3-methyl-6-methoxy-9,10-anthraquinone; CHEMBL42624; ANTHRAQUINONE, 1,8-DIHYDROXY-3-METHOXY-6-METHYL-; H6PT94IV61; NSC-251670; 1,8-dihydroxy-3-methoxy-6-methyl-anthracene-9,10-dione; 9,10-Anthracenedione, 1,8-dihydroxy-3-methoxy-6-methyl-; Methoxyemodin; CCRIS 4399; EINECS 208-315-7; BRN 1915778; fischione; UNII-H6PT94IV61; Physcion,(S); Physcion-[d3]; MFCD00017374; PHYSCIONE-; Spectrum_001782; 9,10-dione; SpecPlus_000471; 6-O-METHYLEMODIN; Rheochrysidin_120098; Spectrum2_000503; Spectrum3_001829; Spectrum4_000909; Spectrum5_001742; Rheochrysidin - Physcione; 1,8-Dihydroxy-3-methoxy-6-methyl-anthraquinone; BSPBio_003477; KBioGR_001378; KBioSS_002265; Physcion, analytical standard; DivK1c_006567; SCHEMBL486155; SPECTRUM1504070; SPBio_000425; MEGxm0_000018; CHEBI:38167; KBio1_001511; KBio2_002264; KBio2_004832; KBio2_007400; KBio3_002981; DTXSID20200101; HMS3656I15; HMS3874M13; Physcion, >=98.0% (TLC); EX-A6797; HY-N0108; ZINC3978794; EMODIN 3-METHYL ETHER [MI]; BDBM50005886; CCG-38713; s2395; AKOS015905689; CHRYSOPHANIC ACID, 6-METHOXY-; AC-7979; KS-5385; SDCCGMLS-0066772.P001; NCGC00096075-01; NCGC00096075-02; NCGC00096075-03; 9, 1,8-dihydroxy-3-methoxy-6-methyl-; AS-15488; NCI60_002011; CS-0007795; FT-0657510; SW219324-1; 1,8-dihydroxy-3-methoxy-6-methylanthracene-; 1,8-dihydroxy-6-methoxy-3-methylanthraquinone; Anthraquinone,8-dihydroxy-3-methoxy-6-methyl-; C17045; 521P619; A828949; SR-05000002537; Q-100587; Q1668551; SR-05000002537-1; 8,9-dihydroxy-3-methyl-6-methoxy-1,10-anthraquinone; 8,9-dihydroxy-6-methyl-3-methoxy-1,10-anthraquinone; BRD-K67772619-001-03-6; 1,8-Dihydroxy-3-methoxy-6-methylanthra-9,10-quinone #; 7-methoxy-2-methyl-4,5-dihydroxyanthracene-9,10-dione; 1,8-Dihydroxy-3-methoxy-6-methylanthraquinone, Emodin-3-methyl ether
|
|
| CAS | 521-61-9 | |
| PubChem CID | 10639 | |
| ChEMBL ID | CHEMBL42624 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 284.26 | ALogp: | 3.0 |
| HBD: | 2 | HBA: | 5 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 83.8 | Aromatic Rings: | 3 |
| Heavy Atoms: | 21 | QED Weighted: | 0.717 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.078 | MDCK Permeability: | 0.00001620 |
| Pgp-inhibitor: | 0.044 | Pgp-substrate: | 0.003 |
| Human Intestinal Absorption (HIA): | 0.021 | 20% Bioavailability (F20%): | 0.027 |
| 30% Bioavailability (F30%): | 0.996 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.045 | Plasma Protein Binding (PPB): | 99.49% |
| Volume Distribution (VD): | 0.429 | Fu: | 1.07% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.947 | CYP1A2-substrate: | 0.835 |
| CYP2C19-inhibitor: | 0.208 | CYP2C19-substrate: | 0.064 |
| CYP2C9-inhibitor: | 0.597 | CYP2C9-substrate: | 0.579 |
| CYP2D6-inhibitor: | 0.343 | CYP2D6-substrate: | 0.267 |
| CYP3A4-inhibitor: | 0.624 | CYP3A4-substrate: | 0.15 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.339 | Half-life (T1/2): | 0.15 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.04 | Human Hepatotoxicity (H-HT): | 0.058 |
| Drug-inuced Liver Injury (DILI): | 0.935 | AMES Toxicity: | 0.856 |
| Rat Oral Acute Toxicity: | 0.207 | Maximum Recommended Daily Dose: | 0.926 |
| Skin Sensitization: | 0.258 | Carcinogencity: | 0.536 |
| Eye Corrosion: | 0.004 | Eye Irritation: | 0.962 |
| Respiratory Toxicity: | 0.123 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
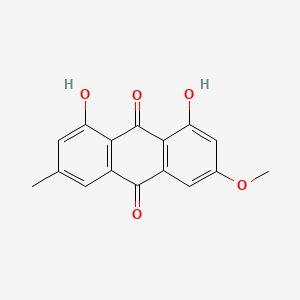
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001497 | 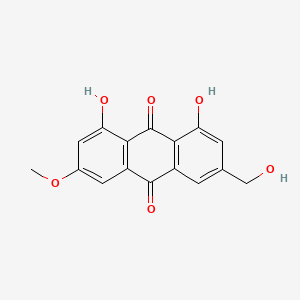 |
0.773 | D0N1FS | 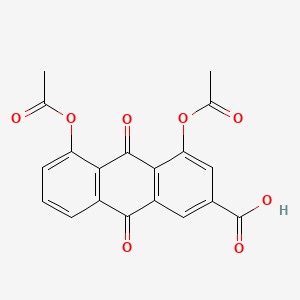 |
0.327 | ||
| ENC000094 | 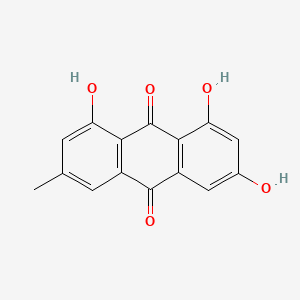 |
0.762 | D07MGA |  |
0.326 | ||
| ENC000939 | 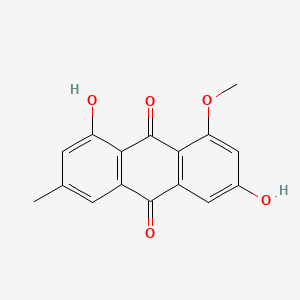 |
0.754 | D06GCK |  |
0.299 | ||
| ENC002229 | 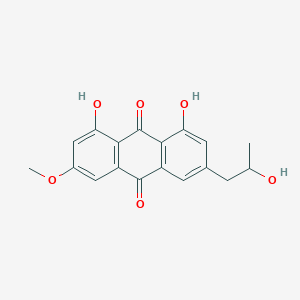 |
0.743 | D04AIT |  |
0.278 | ||
| ENC000930 | 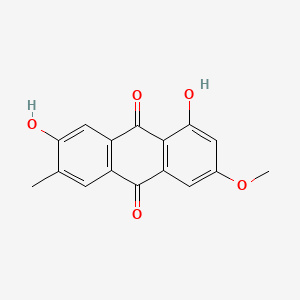 |
0.727 | D0K8KX |  |
0.272 | ||
| ENC005227 | 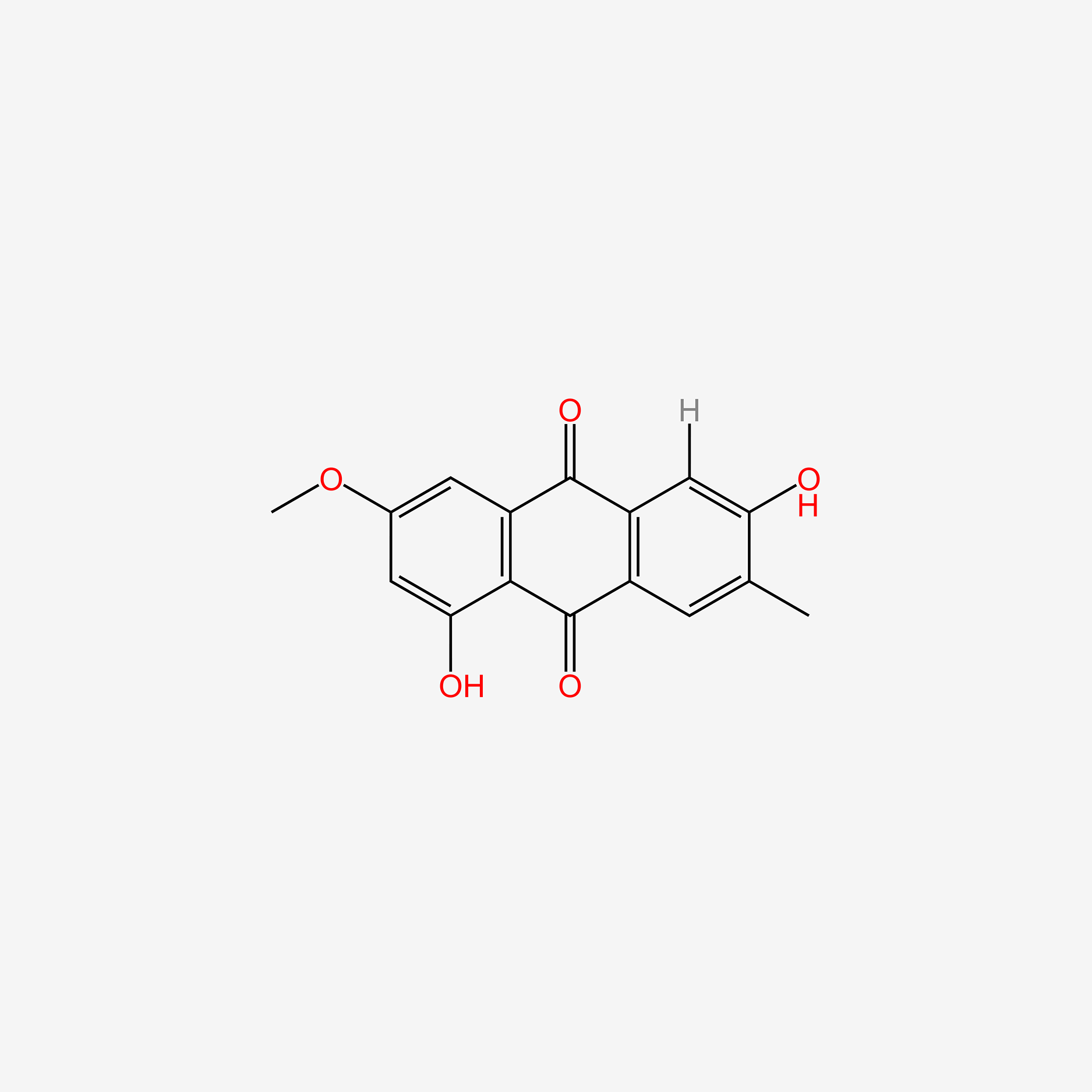 |
0.727 | D0AZ8C |  |
0.260 | ||
| ENC002031 |  |
0.727 | D01XDL |  |
0.250 | ||
| ENC000966 |  |
0.706 | D01XWG | 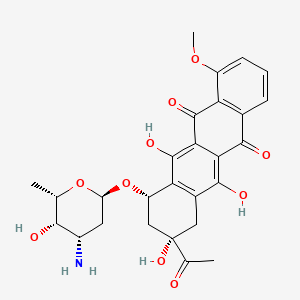 |
0.250 | ||
| ENC000336 | 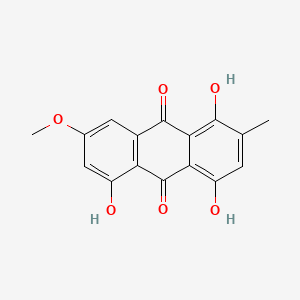 |
0.681 | D09WKB | 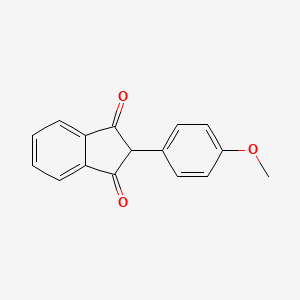 |
0.247 | ||
| ENC005490 | 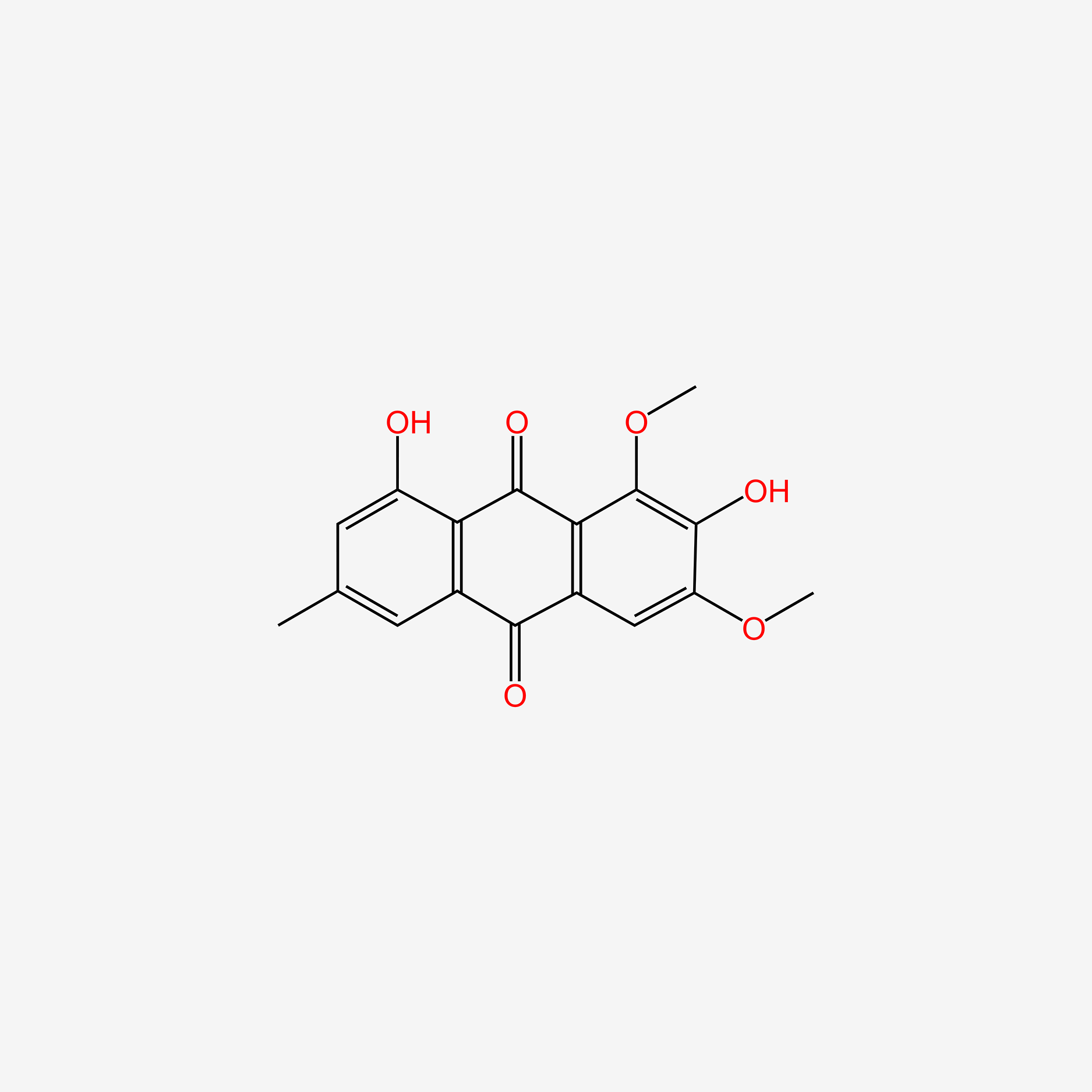 |
0.653 | D0Y7PG |  |
0.247 | ||