NPs Basic Information
|
Name |
Emodin
|
| Molecular Formula | C15H10O5 | |
| IUPAC Name* |
1,3,8-trihydroxy-6-methylanthracene-9,10-dione
|
|
| SMILES |
CC1=CC2=C(C(=C1)O)C(=O)C3=C(C2=O)C=C(C=C3O)O
|
|
| InChI |
InChI=1S/C15H10O5/c1-6-2-8-12(10(17)3-6)15(20)13-9(14(8)19)4-7(16)5-11(13)18/h2-5,16-18H,1H3
|
|
| InChIKey |
RHMXXJGYXNZAPX-UHFFFAOYSA-N
|
|
| Synonyms |
emodin; 518-82-1; Emodol; Frangula emodin; Schuttgelb; 1,3,8-trihydroxy-6-methylanthracene-9,10-dione; Rheum emodin; Archin; Frangulic acid; 3-Methyl-1,6,8-trihydroxyanthraquinone; Persian Berry Lake; 1,3,8-Trihydroxy-6-methylanthraquinone; 6-Methyl-1,3,8-trihydroxyanthraquinone; 1,3,8-Trihydroxy-6-methyl-9,10-anthraquinone; 1,3,8-Trihydroxy-6-methyl-9,10-anthracenedione; C.I. Natural Yellow 14; 9,10-Anthracenedione, 1,3,8-trihydroxy-6-methyl-; Alatinone; Rheum-emodin; 4,5,7-Trihydroxy-2-methylanthraquinone; Frangulinic acid; C.I. 75440; NSC 408120; NSC 622947; Anthraquinone, 1,3,8-trihydroxy-6-methyl-; NSC408120; KA46RNI6HN; CHEMBL289277; Anthraquinone, 6-methyl-1,3,8-trihydroxy-; CHEBI:42223; 1,3,8-Trihydroxy-6-methylanthra-9,10-quinone; NSC622947; NSC-408120; NSC-622947; 1,3,8-trihydroxy-6-methyl-9,10-dihydroanthracene-9,10-dione; DSSTox_CID_5231; DSSTox_RID_77709; 6-Methyl-1,3,8-trihydroxy-9,10-anthracenedione; DSSTox_GSID_25231; EMO; 1,8-Trihydroxy-6-methylanthraquinone; CAS-518-82-1; SMR000326798; CCRIS 3528; HSDB 7093; SR-01000075615; EINECS 208-258-8; UNII-KA46RNI6HN; BRN 1888141; Emdoin; AI3-38286; 3bqc; Emodin,(S); MFCD00001207; Spectrum_001954; 1f0q; 3ed0; SpecPlus_000332; EMODIN [HSDB]; EMODIN [INCI]; 1,3,8-tri-hydroxy-6-methyl-anthra-quinone; EMODIN [USP-RS]; Spectrum2_000895; Spectrum3_000742; Spectrum4_001757; Spectrum5_000614; EMODIN [MI]; Lopac-E-7881; Emodin, analytical standard; NCIMech_000049; Lopac0_000552; BSPBio_002324; KBioGR_002234; KBioSS_002508; 1,3,8-trihydroxy-6-methyl-anthracene-9,10-dione; MLS000563068; MLS001066370; MLS004257392; MLS006011712; DivK1c_006428; SCHEMBL177689; SPBio_000710; MEGxp0_000460; DTXSID5025231; ACon1_001939; BDBM11318; KBio1_001372; KBio2_002500; KBio2_005068; KBio2_007636; KBio3_001544; Emodin - CAS 518-82-1; HMS2230K22; HMS3261P05; HMS3373B16; HMS3655H22; ACT03256; BCP18372; EX-A6778; TNP00318; ZINC3824868; 9, 1,3,8-trihydroxy-6-methyl-; Tox21_202999; Tox21_303218; Tox21_500552; CCG-35263; LMPK13040008; s2295; STL581876; 3-Methyl-1,8-trihydroxyanthraquinone; 4,7-Trihydroxy-2-methylanthraquinone; AKOS003348641; AC-1004; CS-1412; DB07715; KS-5189; LP00552; SDCCGSBI-0050535.P004; Anthraquinone,3,8-trihydroxy-6-methyl-; SMP2_000211; NCGC00015420-01; NCGC00015420-02; NCGC00015420-03; NCGC00015420-04; NCGC00015420-05; NCGC00015420-06; NCGC00015420-07; NCGC00015420-08; NCGC00015420-09; NCGC00015420-22; NCGC00091540-01; NCGC00091540-02; NCGC00091540-03; NCGC00091540-04; NCGC00091540-05; NCGC00257090-01; NCGC00260544-01; NCGC00261237-01; 1,3,8-Trihydroxy-6-methyl-anthraquinone; HY-14393; NCI60_003906; E0500; EU-0100552; FT-0606539; FT-0667846; SW219906-1; 1,8-Trihydroxy-6-methyl-9,10-anthraquinone; Emodin, from Frangula bark, >=90% (HPLC); E 7881; EN300-179559; K00056; Emodin; 6-Methyl-1,3,8-trihydroxyanthraquinone; 1,3, 8-Trihydroxy-6-methyl-9,10-anthraquinone; 1,3,8-Trihydroxy-6-methylanthra-9,10-quinone #; 518E821; A828825; Q-100581; Q4348178; SR-01000075615-1; SR-01000075615-6; BRD-K58685305-001-03-0; 1,3,8-Trihydroxy-6-methyl-9,10-anthracenedione, 9CI; 9,10-Anthracenedione, 1,3,8-trihydroxy-6-methyl- (9CI); Emodin, United States Pharmacopeia (USP) Reference Standard; 1,3,8-trihydroxy-6-methyl-anthracene-9,10-dione;3-METHYL-1,6,8-TRIHYDROXYANTHRAQUINONE
|
|
| CAS | 518-82-1 | |
| PubChem CID | 3220 | |
| ChEMBL ID | CHEMBL289277 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 270.24 | ALogp: | 2.7 |
| HBD: | 3 | HBA: | 5 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 94.8 | Aromatic Rings: | 3 |
| Heavy Atoms: | 20 | QED Weighted: | 0.583 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.149 | MDCK Permeability: | 0.00001100 |
| Pgp-inhibitor: | 0.014 | Pgp-substrate: | 0.006 |
| Human Intestinal Absorption (HIA): | 0.016 | 20% Bioavailability (F20%): | 0.82 |
| 30% Bioavailability (F30%): | 0.999 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.056 | Plasma Protein Binding (PPB): | 99.29% |
| Volume Distribution (VD): | 0.482 | Fu: | 1.64% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.95 | CYP1A2-substrate: | 0.338 |
| CYP2C19-inhibitor: | 0.103 | CYP2C19-substrate: | 0.054 |
| CYP2C9-inhibitor: | 0.575 | CYP2C9-substrate: | 0.479 |
| CYP2D6-inhibitor: | 0.378 | CYP2D6-substrate: | 0.184 |
| CYP3A4-inhibitor: | 0.651 | CYP3A4-substrate: | 0.114 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 9.245 | Half-life (T1/2): | 0.385 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.039 | Human Hepatotoxicity (H-HT): | 0.046 |
| Drug-inuced Liver Injury (DILI): | 0.935 | AMES Toxicity: | 0.823 |
| Rat Oral Acute Toxicity: | 0.096 | Maximum Recommended Daily Dose: | 0.916 |
| Skin Sensitization: | 0.678 | Carcinogencity: | 0.301 |
| Eye Corrosion: | 0.005 | Eye Irritation: | 0.961 |
| Respiratory Toxicity: | 0.076 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
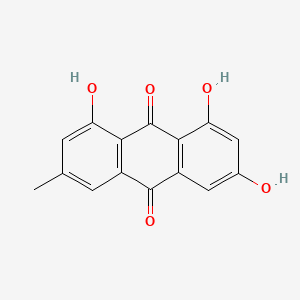
| ENC000362 | 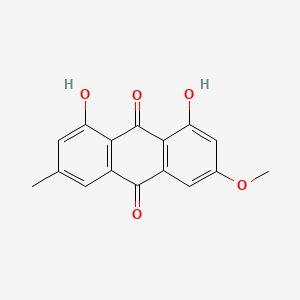 |
0.762 | D07MGA |  |
0.369 | ||
| ENC000939 | 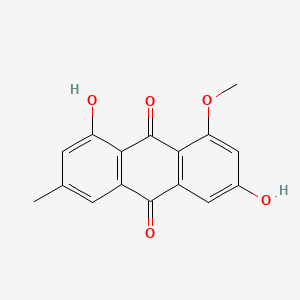 |
0.762 | D04AIT |  |
0.366 | ||
| ENC001058 |  |
0.762 | D0K8KX |  |
0.357 | ||
| ENC002031 | 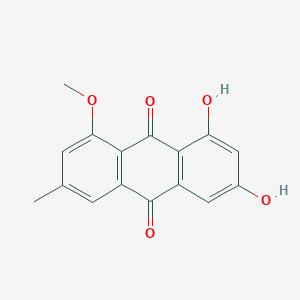 |
0.762 | D0N1FS | 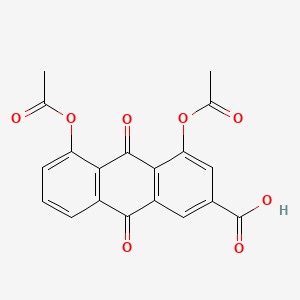 |
0.309 | ||
| ENC002296 | 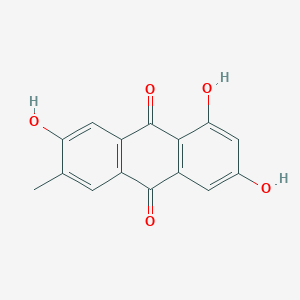 |
0.714 | D0AZ8C | 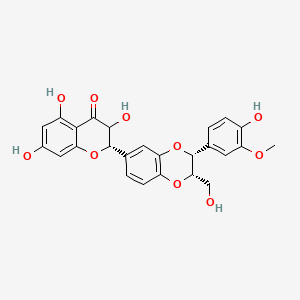 |
0.288 | ||
| ENC000337 | 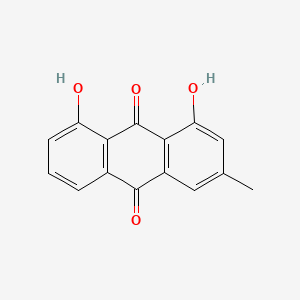 |
0.683 | D07EXH | 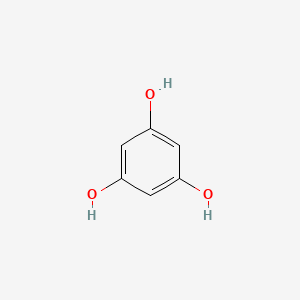 |
0.279 | ||
| ENC001929 | 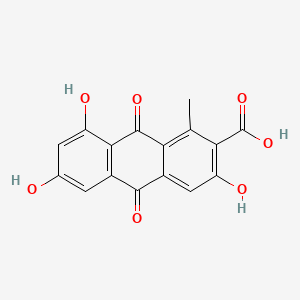 |
0.667 | D0R3JB | 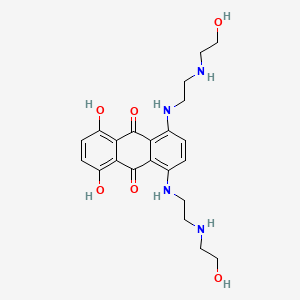 |
0.263 | ||
| ENC000335 | 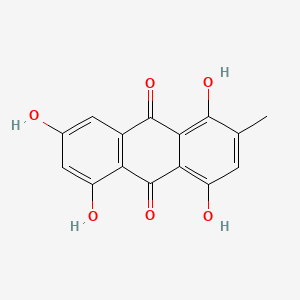 |
0.667 | D0Y7PG | 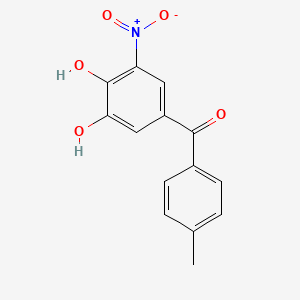 |
0.256 | ||
| ENC001971 | 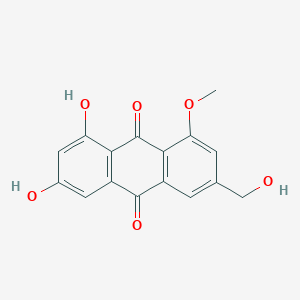 |
0.652 | D06GCK |  |
0.255 | ||
| ENC005602 | 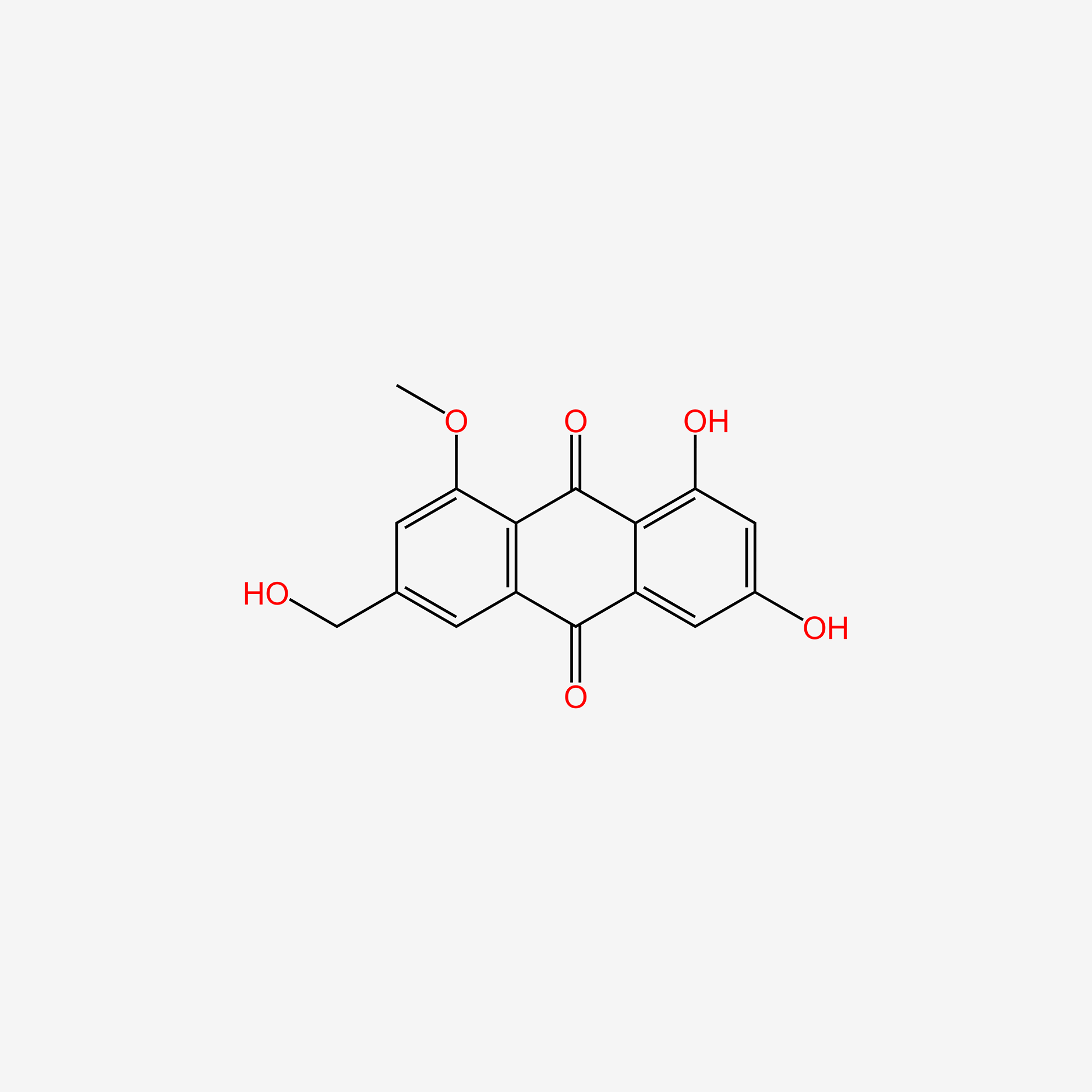 |
0.652 | D03GET | 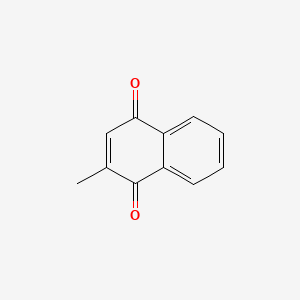 |
0.250 | ||