NPs Basic Information
|
Name |
Xanthoxylin
|
| Molecular Formula | C10H12O4 | |
| IUPAC Name* |
1-(2-hydroxy-4,6-dimethoxyphenyl)ethanone
|
|
| SMILES |
CC(=O)C1=C(C=C(C=C1OC)OC)O
|
|
| InChI |
InChI=1S/C10H12O4/c1-6(11)10-8(12)4-7(13-2)5-9(10)14-3/h4-5,12H,1-3H3
|
|
| InChIKey |
FBUBVLUPUDBFME-UHFFFAOYSA-N
|
|
| Synonyms |
Xanthoxylin; 90-24-4; Xanthoxyline; 2'-Hydroxy-4',6'-dimethoxyacetophenone; Brevifolin; 1-(2-Hydroxy-4,6-dimethoxyphenyl)ethanone; 2-Hydroxy-4,6-dimethoxyacetophenone; 1-(2-Hydroxy-4,6-dimethoxyphenyl)ethan-1-one; Brevifolin (Zanthoxylum); 2,4-Di-O-methylphloroacetophenone; Phloracetophenone dimethyl ether; Phloroacetophenone 2,4-dimethyl ether; Brevifolin (VAN); 4',6'-dimethoxy-2'-hydroxyacetophenone; 4,6-Dimethoxy-2-hydroxyacetophenone; Ethanone, 1-(2-hydroxy-4,6-dimethoxyphenyl)-; 1-Acetyl-2-hydroxy-4,6-dimethoxybenzene; Acetophenone der.; Z8RSY5TZPA; NSC 17392; 2-Acetyl-3,5-dimethoxyphenol; Acetophenone, 2'-hydroxy-4',6'-dimethoxy-; CHEBI:10070; MFCD00017243; NSC-17392; 2-Hydroxyl-4,6-dimethoxy-acetophenone; 1-(2-hydroxy-4,6-dimethoxy-phenyl)ethanone; Ethanone,1-(2-hydroxy-4,6-dimethoxyphenyl)-; UNII-Z8RSY5TZPA; 6-Methoxypaeonol; EINECS 201-978-3; Spectrum_000577; SpecPlus_000713; AI3-26010; XANTHOXYLIN [MI]; Spectrum2_000463; Spectrum3_000181; Spectrum4_001499; Spectrum5_000237; Acetophenone,6'-dimethoxy-; SCHEMBL44708; BSPBio_001701; KBioGR_002137; KBioSS_001057; SPECTRUM200441; MLS002207182; DivK1c_006809; XANTHOXYLINE [WHO-DD]; SPBio_000566; CHEMBL450288; KBio1_001753; KBio2_001057; KBio2_003625; KBio2_006193; KBio3_001201; DTXSID10237981; ZINC157077; HY-N1063; NSC17392; CCG-38702; s4781; 2-Hydroxy-4, 6-dimethoxyacetophenone; 4, 6-Dimethoxy-2-hydroxyacetophenone; AKOS015856339; SDCCGMLS-0066937.P001; 2'-hyroxy-4',6'-dimethoxyacetophenone; PHLOROACETOPHENONE DIMETHYL ETHER; NCGC00095824-01; NCGC00095824-02; AC-35124; AS-40799; SMR001306755; SY048579; 2'-Hydroxy-4',6'-dimethoxy-Acetophenone; (2-hydroxy-4,6-dimethoxy-phenyl)-ethanone; 1-(2-hydroxy-4,6-dimethoxyphenyl)-ethanone; 1-(2-Hydroxy-4,6-dimethylphenyl)-ethanone; CS-0016347; D2683; FT-0612544; 1-(2-Hydroxy-4,6-dimethoxyphenyl)ethanone #; 2'-Hydroxy-4',6'-dimethoxyacetophenone, 97%; 2',4'-DIMETHOXY-6'-HYDROXYACETOPHENONE; EN300-6477862; A843476; AQ-358/42007313; SR-05000002434; 2''-HYDROXY-4'',6''-DIMETHOXYACETOPHENONE; Acetophenone, 2'-hydroxy-4',6'-dimethoxy- (8CI); SR-05000002434-1; BRD-K12260308-001-02-6; BRD-K12260308-001-04-2; Q18210424; Z1255434817; 2 inverted exclamation mark -Hydroxy-4 inverted exclamation mark ,6 inverted exclamation mark -dimethoxyacetophenone
|
|
| CAS | 90-24-4 | |
| PubChem CID | 66654 | |
| ChEMBL ID | CHEMBL450288 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 196.2 | ALogp: | 1.7 |
| HBD: | 1 | HBA: | 4 |
| Rotatable Bonds: | 3 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 55.8 | Aromatic Rings: | 1 |
| Heavy Atoms: | 14 | QED Weighted: | 0.753 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.642 | MDCK Permeability: | 0.00001630 |
| Pgp-inhibitor: | 0.002 | Pgp-substrate: | 0.003 |
| Human Intestinal Absorption (HIA): | 0.012 | 20% Bioavailability (F20%): | 0.006 |
| 30% Bioavailability (F30%): | 0.047 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.78 | Plasma Protein Binding (PPB): | 83.70% |
| Volume Distribution (VD): | 0.95 | Fu: | 18.75% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.966 | CYP1A2-substrate: | 0.924 |
| CYP2C19-inhibitor: | 0.403 | CYP2C19-substrate: | 0.72 |
| CYP2C9-inhibitor: | 0.201 | CYP2C9-substrate: | 0.905 |
| CYP2D6-inhibitor: | 0.426 | CYP2D6-substrate: | 0.881 |
| CYP3A4-inhibitor: | 0.333 | CYP3A4-substrate: | 0.319 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 9.963 | Half-life (T1/2): | 0.66 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.037 | Human Hepatotoxicity (H-HT): | 0.069 |
| Drug-inuced Liver Injury (DILI): | 0.658 | AMES Toxicity: | 0.136 |
| Rat Oral Acute Toxicity: | 0.387 | Maximum Recommended Daily Dose: | 0.108 |
| Skin Sensitization: | 0.274 | Carcinogencity: | 0.041 |
| Eye Corrosion: | 0.269 | Eye Irritation: | 0.984 |
| Respiratory Toxicity: | 0.869 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
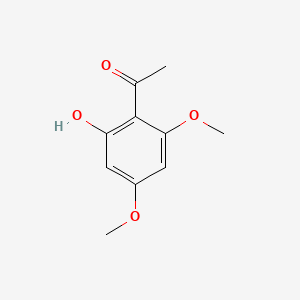
| ENC002382 |  |
0.549 | D09GYT | 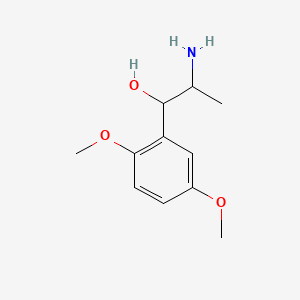 |
0.351 | ||
| ENC004779 | 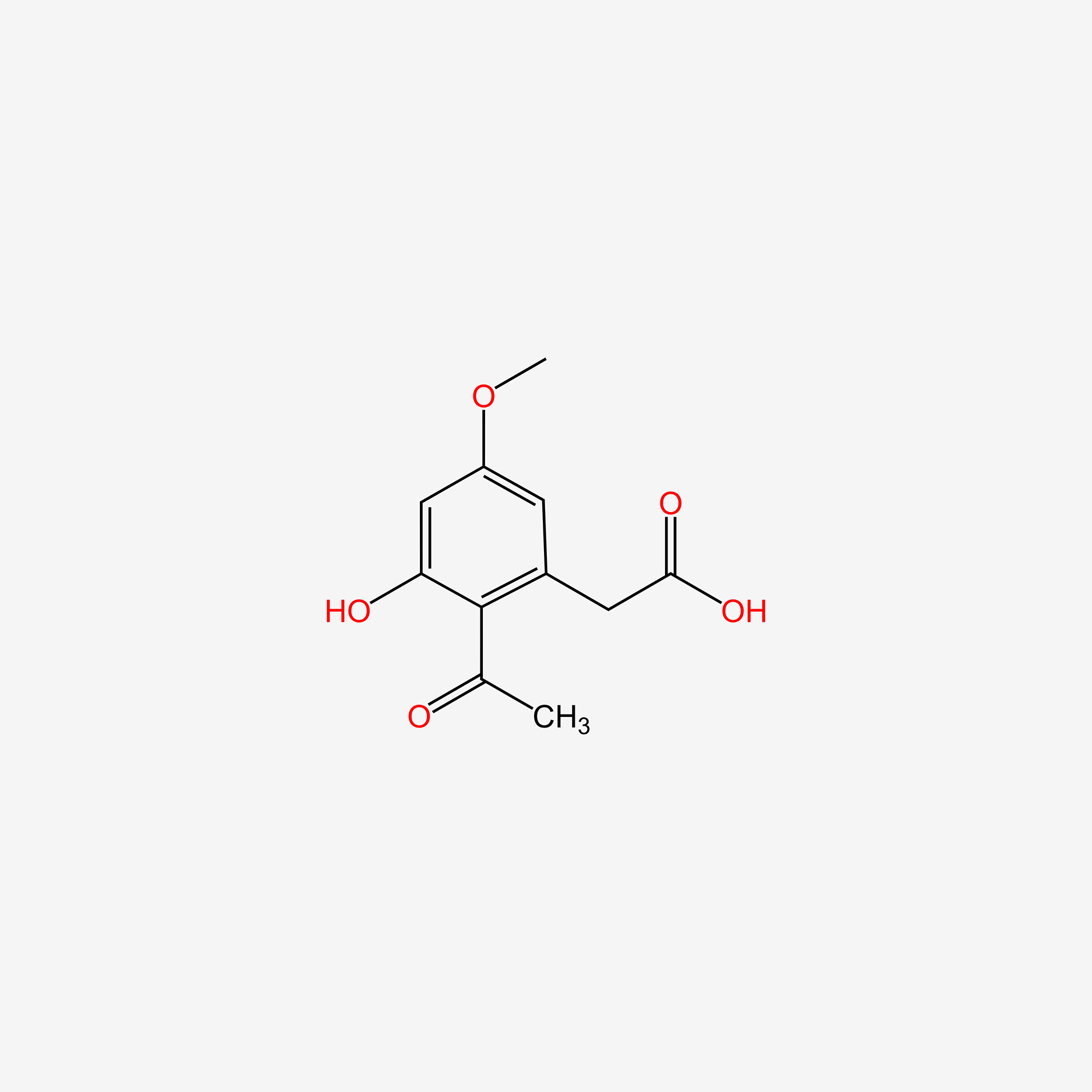 |
0.549 | D02XJY | 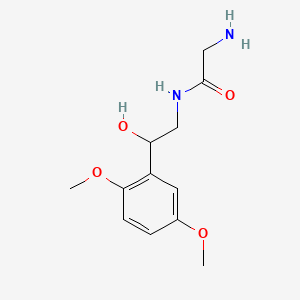 |
0.344 | ||
| ENC002461 | 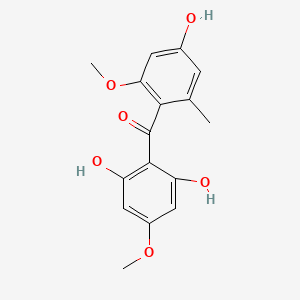 |
0.500 | D0E9CD |  |
0.314 | ||
| ENC001379 | 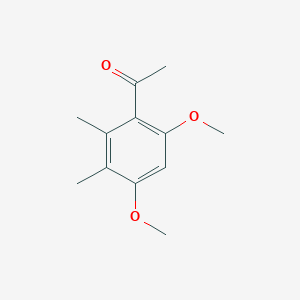 |
0.490 | D0DJ1B |  |
0.292 | ||
| ENC004806 | 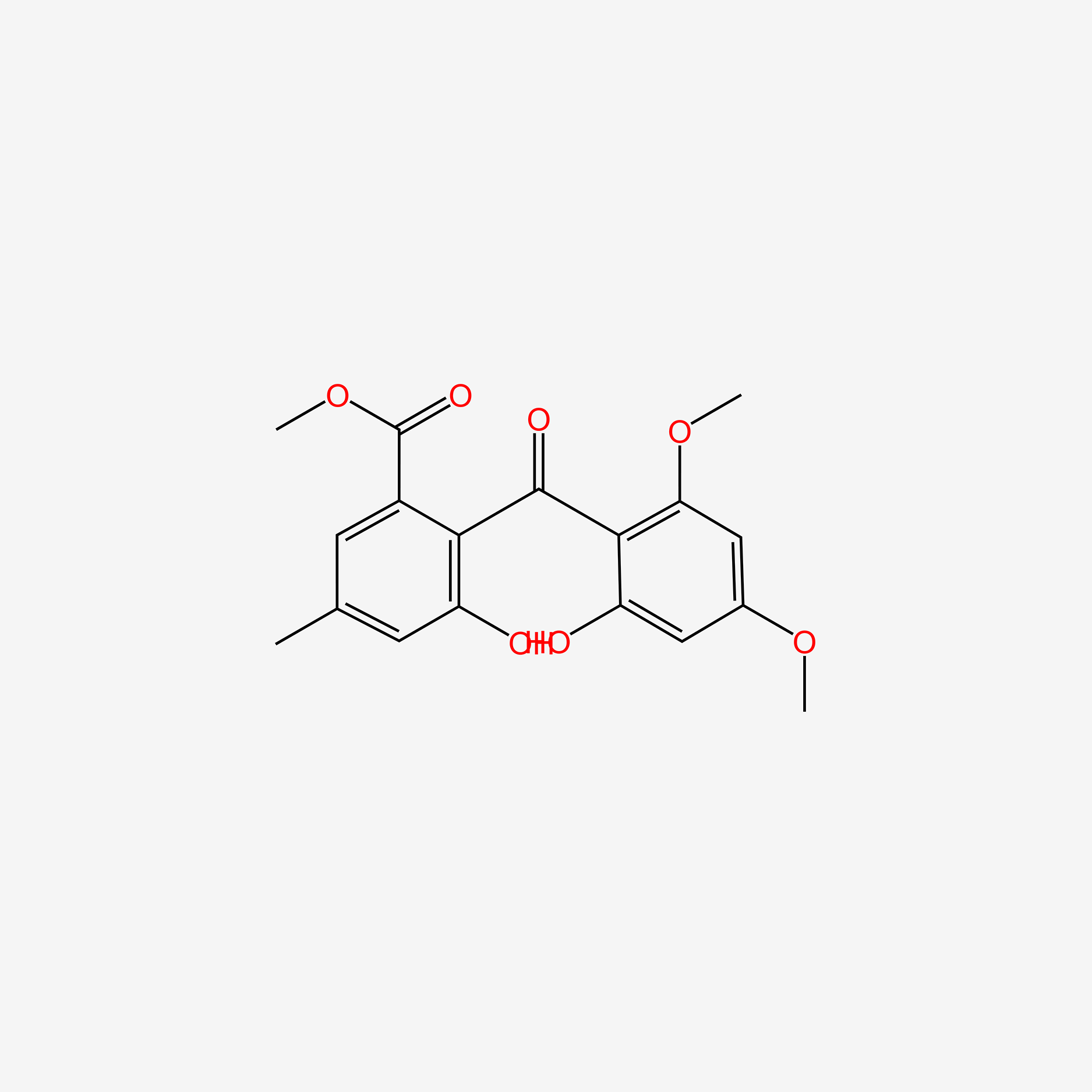 |
0.465 | D05CKR | 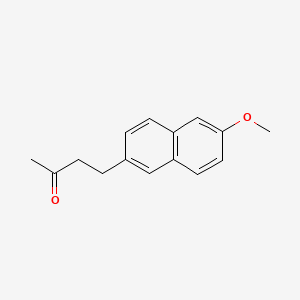 |
0.288 | ||
| ENC000367 | 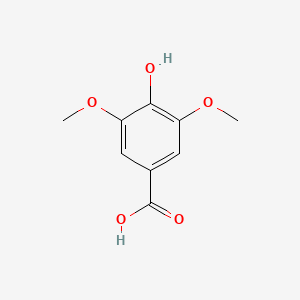 |
0.451 | D06GCK |  |
0.277 | ||
| ENC004830 | 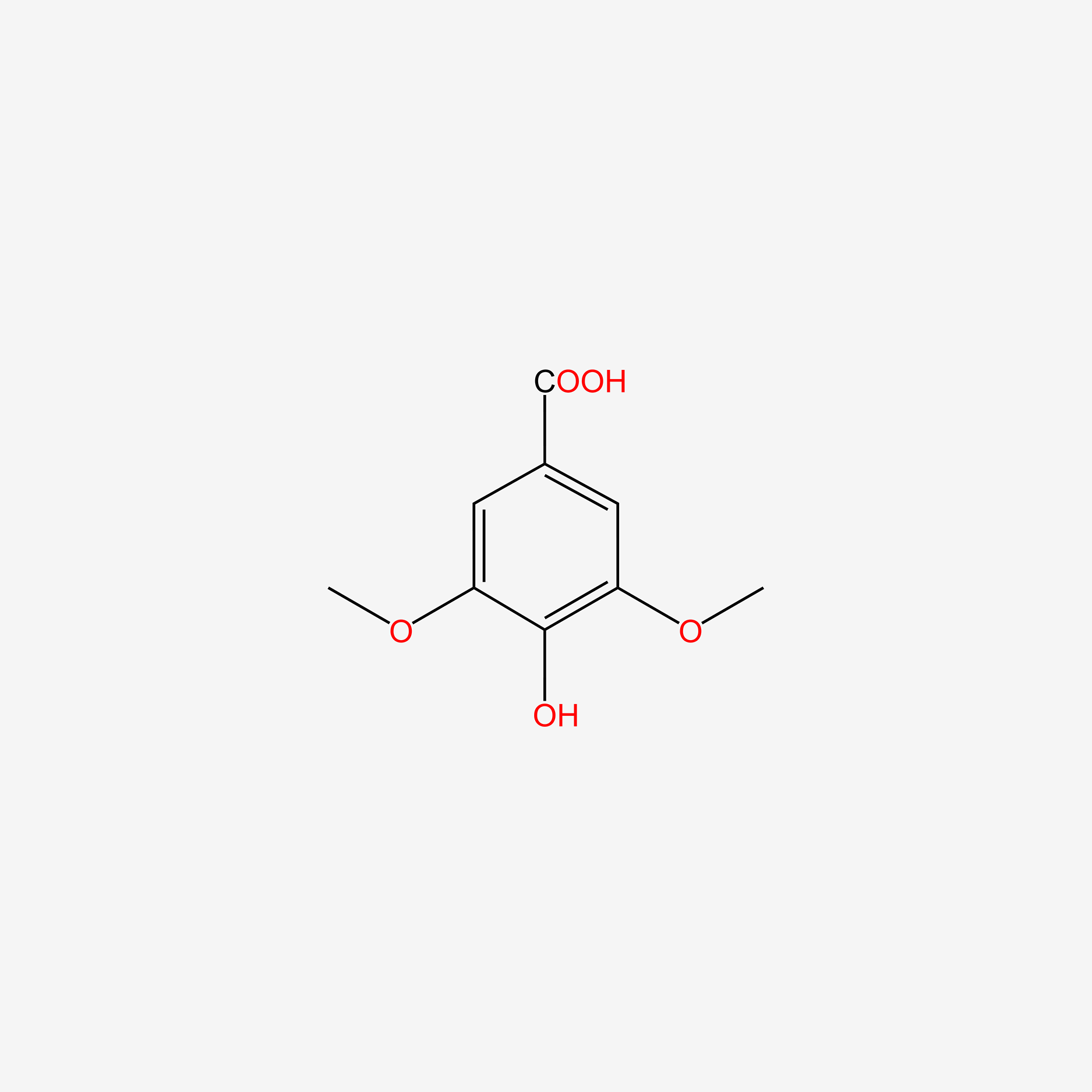 |
0.451 | D0AN7B | 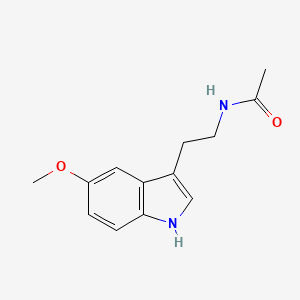 |
0.269 | ||
| ENC005979 | 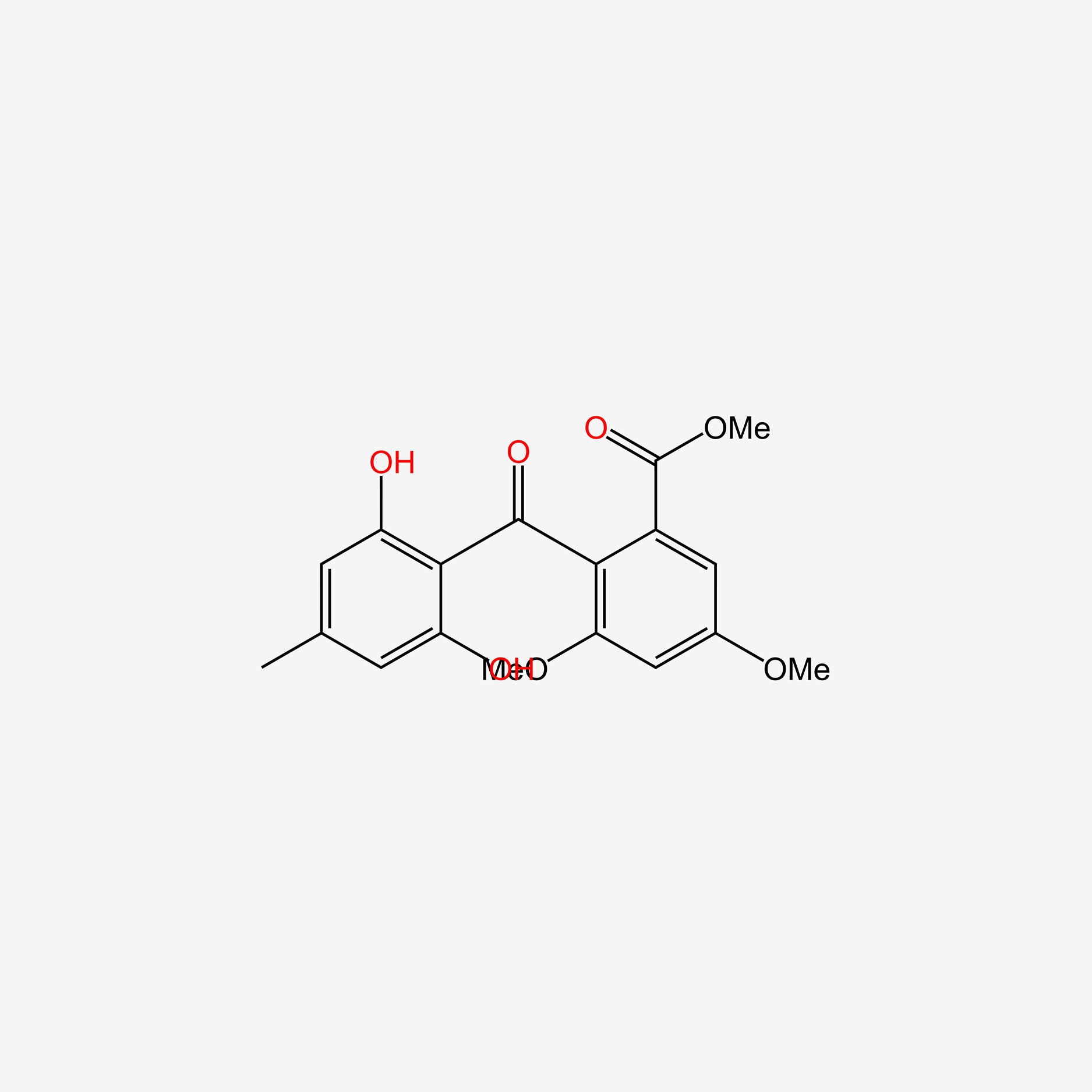 |
0.444 | D08VYV | 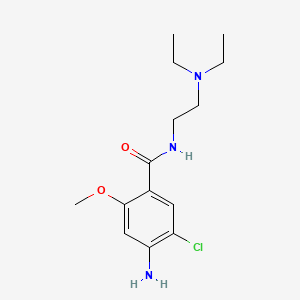 |
0.264 | ||
| ENC000478 | 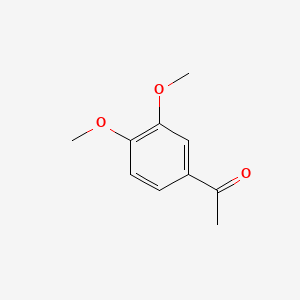 |
0.440 | D00WVW | 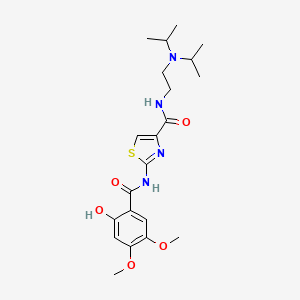 |
0.260 | ||
| ENC000764 | 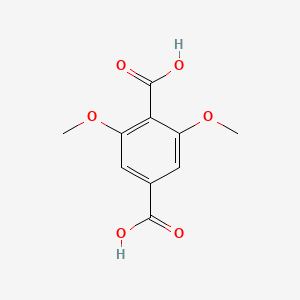 |
0.436 | D06TQZ | 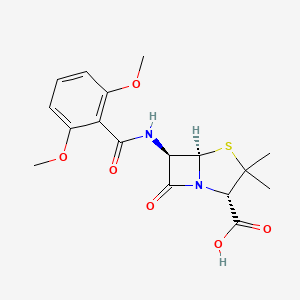 |
0.259 | ||