NPs Basic Information
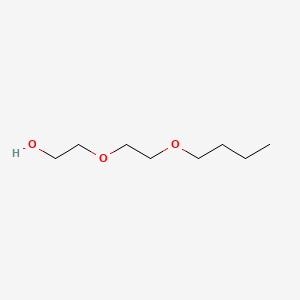
|
Name |
2-(2-Butoxyethoxy)ethanol
|
| Molecular Formula | C8H18O3 | |
| IUPAC Name* |
2-(2-butoxyethoxy)ethanol
|
|
| SMILES |
CCCCOCCOCCO
|
|
| InChI |
InChI=1S/C8H18O3/c1-2-3-5-10-7-8-11-6-4-9/h9H,2-8H2,1H3
|
|
| InChIKey |
OAYXUHPQHDHDDZ-UHFFFAOYSA-N
|
|
| Synonyms |
2-(2-Butoxyethoxy)ethanol; 112-34-5; Butyldiglycol; DIETHYLENE GLYCOL MONOBUTYL ETHER; Butyl carbitol; Diethylene glycol butyl ether; Butoxydiglycol; Ethanol, 2-(2-butoxyethoxy)-; Butyl diglycol; Butyl dioxitol; Butyl digol; Butoxyethoxyethanol; BUCB; Dowanol DB; Glycol ether DB; Jeffersol db; Ektasolve DB; Butoxydiethylene glycol; Diglycol monobutyl ether; O-Butyl diethylene glycol; Diethylene glycol mono-n-butyl ether; Butoxy diethylene glycol; Diethylene glycol n-butyl ether; Diethylene gylcol monobutyl ether; NSC 407762; Ethanol, 2,2'-oxybis-, monobutyl ether; Monobutyl diethylene glycol ether; 9TB90IYC0E; 2-(2-butoxyethoxy)-ethanol; 2-(2-n-Butoxyethoxy)ethanol; NSC-407762; DSSTox_CID_1519; DSSTox_RID_76196; DSSTox_GSID_21519; Caswell No. 121B; Caswell No. 125H; n-Butyl carbitol; Diethylene glycol butyl ether, >=99%; 3,6-Dioxadecanol; CAS-112-34-5; CCRIS 5321; HSDB 333; 2-(2-butoxyethoxy)ethan-1-ol; 3,6-Dioxa-1-decanol; EINECS 203-961-6; UNII-9TB90IYC0E; EPA Pesticide Chemical Code 011502; BRN 1739225; Butadigol; AI3-01954; Butyl di-icinol; Diethylene DB; DEGBE; Ethanol 2-butoxyethoxy; Butyl Oxitol glycol ether; 2-(n-Butoxyethoxy)ethanol; EC 203-961-6; SCHEMBL15619; 2-(2-Butoxyethoxy);ethanol; BUTOXYDIGLYCOL [INCI]; Diethylene glycol butyl ester; diethyleneglycol monobutylether; diethyleneglycol n-butyl ether; WLN: Q2O2O4; diethyleneglycol monobutyl ether; CHEMBL1904721; diethylene glycol-monobutyl ether; DTXSID8021519; ZINC1600070; Tox21_202404; Tox21_300084; Ethanol,2'-oxybis-, monobutyl ether; MFCD00002881; NSC407762; AKOS009156535; Diethylene glycol monobutyl ether, 98%; NCGC00164235-01; NCGC00164235-02; NCGC00164235-03; NCGC00253937-01; NCGC00259953-01; LS-13547; B0699; FT-0624889; DIETHYLENE GLYCOL MONOBUTYL ETHER [MI]; Diethylene Glycol Monobutyl Ether Reagent Grade; EN300-206638; F71187; A802556; DIETHYLENE GLYCOL MONO-N-BUTYL ETHER [HSDB]; Diethylene glycol monobutyl ether, >=98.0% (GC); J-002756; J-519970; Q1018210; Diethylene glycol butyl ether, SAJ special grade, >=99.0%; Diethylene glycol monobutyl ether, for surfactant analysis, >=99.0%
|
|
| CAS | 112-34-5 | |
| PubChem CID | 8177 | |
| ChEMBL ID | CHEMBL1904721 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 162.23 | ALogp: | 0.6 |
| HBD: | 1 | HBA: | 3 |
| Rotatable Bonds: | 8 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 38.7 | Aromatic Rings: | 0 |
| Heavy Atoms: | 11 | QED Weighted: | 0.545 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.163 | MDCK Permeability: | 0.00003420 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.004 |
| Human Intestinal Absorption (HIA): | 0.001 | 20% Bioavailability (F20%): | 0.561 |
| 30% Bioavailability (F30%): | 0.056 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.579 | Plasma Protein Binding (PPB): | 17.69% |
| Volume Distribution (VD): | 0.939 | Fu: | 68.21% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.035 | CYP1A2-substrate: | 0.138 |
| CYP2C19-inhibitor: | 0.024 | CYP2C19-substrate: | 0.428 |
| CYP2C9-inhibitor: | 0.01 | CYP2C9-substrate: | 0.046 |
| CYP2D6-inhibitor: | 0.003 | CYP2D6-substrate: | 0.07 |
| CYP3A4-inhibitor: | 0.006 | CYP3A4-substrate: | 0.148 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.606 | Half-life (T1/2): | 0.788 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.267 | Human Hepatotoxicity (H-HT): | 0.027 |
| Drug-inuced Liver Injury (DILI): | 0.014 | AMES Toxicity: | 0.063 |
| Rat Oral Acute Toxicity: | 0.052 | Maximum Recommended Daily Dose: | 0.006 |
| Skin Sensitization: | 0.676 | Carcinogencity: | 0.531 |
| Eye Corrosion: | 0.517 | Eye Irritation: | 0.988 |
| Respiratory Toxicity: | 0.015 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000605 |  |
0.591 | D01QLH |  |
0.262 | ||
| ENC000264 |  |
0.488 | D05ZPL |  |
0.252 | ||
| ENC000855 |  |
0.439 | D0AY9Q |  |
0.237 | ||
| ENC000049 |  |
0.366 | D0H2SY | 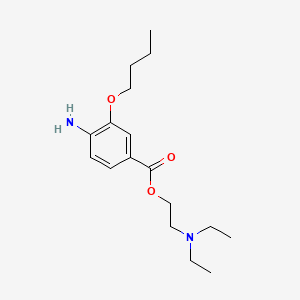 |
0.227 | ||
| ENC000854 | 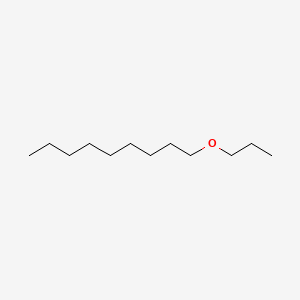 |
0.360 | D05ATI |  |
0.219 | ||
| ENC000279 | 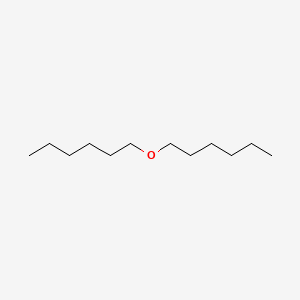 |
0.360 | D09CGE | 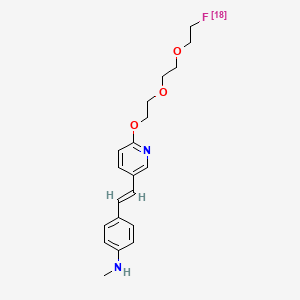 |
0.216 | ||
| ENC000776 |  |
0.359 | D0L0GM |  |
0.208 | ||
| ENC000139 |  |
0.343 | D0V4UF |  |
0.208 | ||
| ENC000317 | 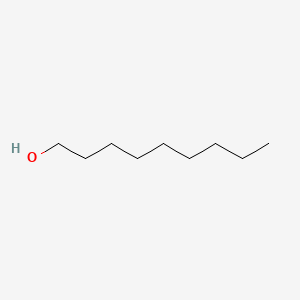 |
0.341 | D02HXS | 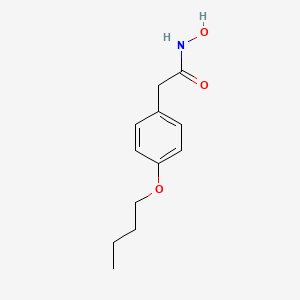 |
0.206 | ||
| ENC000017 | 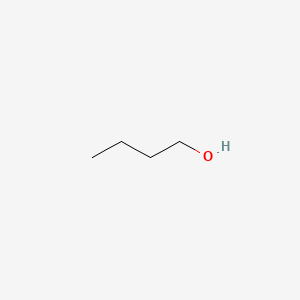 |
0.333 | D07ILQ |  |
0.197 | ||