NPs Basic Information
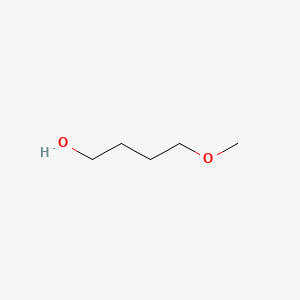
|
Name |
4-Methoxybutan-1-ol
|
| Molecular Formula | C5H12O2 | |
| IUPAC Name* |
4-methoxybutan-1-ol
|
|
| SMILES |
COCCCCO
|
|
| InChI |
InChI=1S/C5H12O2/c1-7-5-3-2-4-6/h6H,2-5H2,1H3
|
|
| InChIKey |
KOVAQMSVARJMPH-UHFFFAOYSA-N
|
|
| Synonyms |
4-Methoxybutan-1-ol; 111-32-0; 4-METHOXY-1-BUTANOL; 1-Butanol, 4-methoxy-; Dowanol BMAT; Dowanol bm; Butylene glycol methyl ether; 4-Methoxybutyl alcohol; 1,4-Butanediol Monomethyl Ether; 4-Methoxybutanol; Butylene glycol monomethyl ether; NSC 245191; AH4W8HA8JH; Tetramethylene Glycol Monomethyl Ether; NSC-245191; Dowanal BMAT; 4-Methoxybutanol-1; EINECS 203-858-6; BRN 1732309; 4-methoxy-butan-1-ol; UNII-AH4W8HA8JH; SCHEMBL23986; WLN: Q4O1; DTXSID50149438; BCP23225; ZINC1765882; BBL100500; MFCD00040437; NSC245191; STL554294; AKOS005254381; WT82446; MS-20025; DB-002112; AM20080057; B2402; CS-0150179; FT-0656133; EN300-42852; D78159; Q-103486; Z431605254
|
|
| CAS | 111-32-0 | |
| PubChem CID | 8107 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 104.15 | ALogp: | 0.1 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 29.5 | Aromatic Rings: | 0 |
| Heavy Atoms: | 7 | QED Weighted: | 0.534 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.193 | MDCK Permeability: | 0.00004230 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.047 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.017 |
| 30% Bioavailability (F30%): | 0.016 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.393 | Plasma Protein Binding (PPB): | 8.41% |
| Volume Distribution (VD): | 0.891 | Fu: | 88.41% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.032 | CYP1A2-substrate: | 0.859 |
| CYP2C19-inhibitor: | 0.019 | CYP2C19-substrate: | 0.649 |
| CYP2C9-inhibitor: | 0.004 | CYP2C9-substrate: | 0.095 |
| CYP2D6-inhibitor: | 0.003 | CYP2D6-substrate: | 0.13 |
| CYP3A4-inhibitor: | 0.005 | CYP3A4-substrate: | 0.185 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.324 | Half-life (T1/2): | 0.768 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.044 | Human Hepatotoxicity (H-HT): | 0.042 |
| Drug-inuced Liver Injury (DILI): | 0.027 | AMES Toxicity: | 0.014 |
| Rat Oral Acute Toxicity: | 0.029 | Maximum Recommended Daily Dose: | 0.016 |
| Skin Sensitization: | 0.626 | Carcinogencity: | 0.359 |
| Eye Corrosion: | 0.902 | Eye Irritation: | 0.989 |
| Respiratory Toxicity: | 0.024 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000139 | 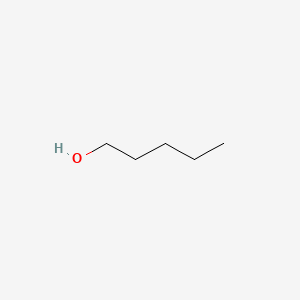 |
0.522 | D01QLH | 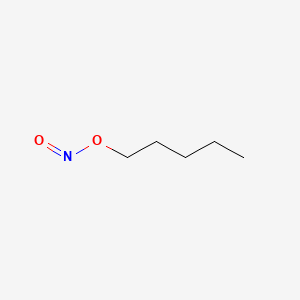 |
0.323 | ||
| ENC000255 | 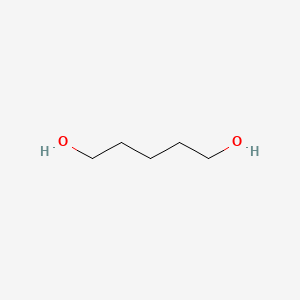 |
0.407 | D0EP8X | 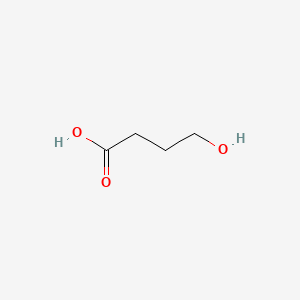 |
0.276 | ||
| ENC000017 | 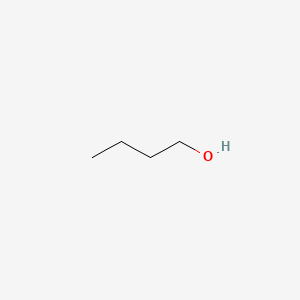 |
0.391 | D03CHT | 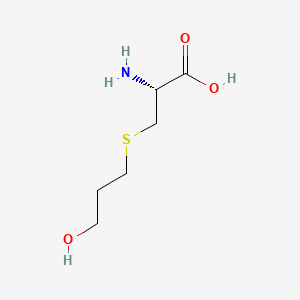 |
0.231 | ||
| ENC000049 | 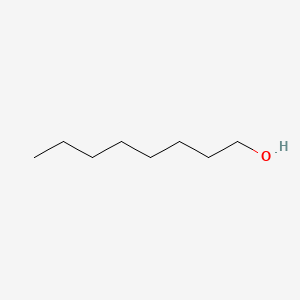 |
0.375 | D07SUG | 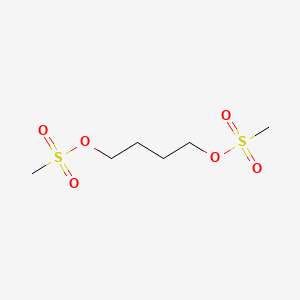 |
0.222 | ||
| ENC000355 | 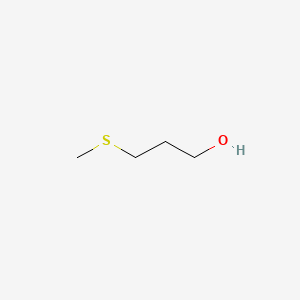 |
0.346 | D03HFG | 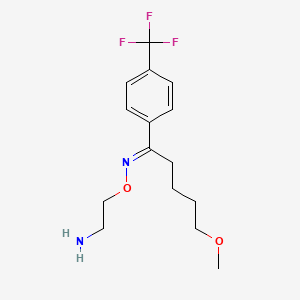 |
0.212 | ||
| ENC000317 | 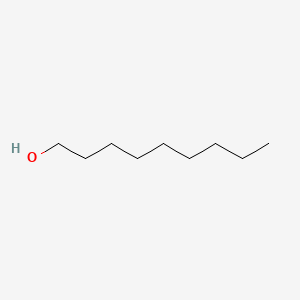 |
0.343 | D0C1QZ | 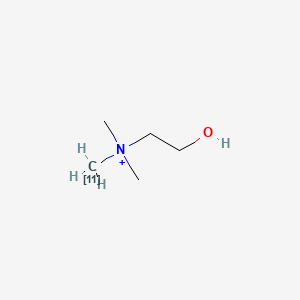 |
0.200 | ||
| ENC000897 | 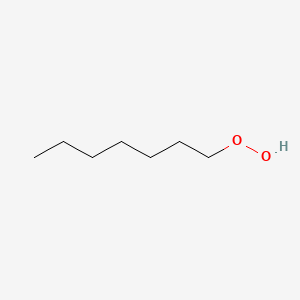 |
0.333 | D0MM8N | 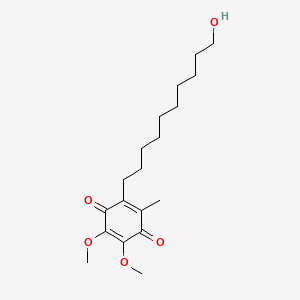 |
0.197 | ||
| ENC000776 | 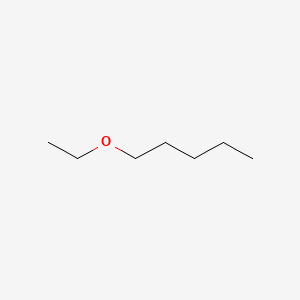 |
0.323 | D0AY9Q | 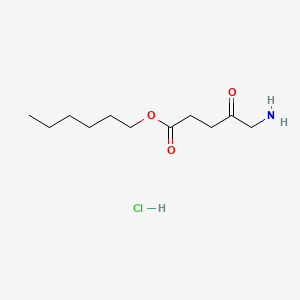 |
0.196 | ||
| ENC000274 | 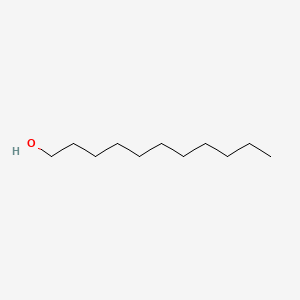 |
0.293 | D0FD0H | 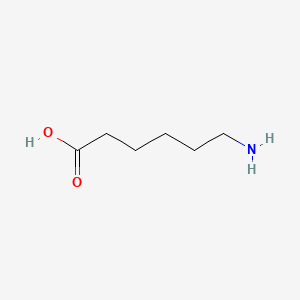 |
0.194 | ||
| ENC003693 | 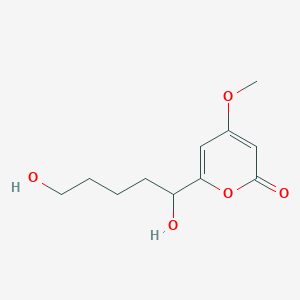 |
0.286 | D00AMQ | 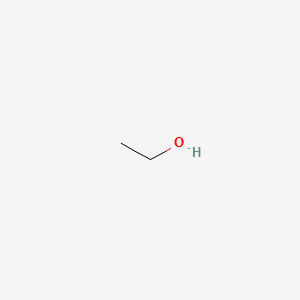 |
0.190 | ||