NPs Basic Information
|
Name |
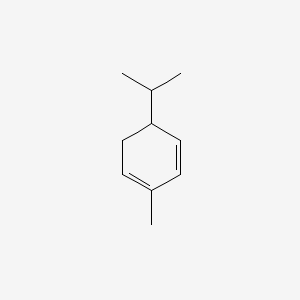
alpha-PHELLANDRENE
|
| Molecular Formula | C10H16 | |
| IUPAC Name* |
2-methyl-5-propan-2-ylcyclohexa-1,3-diene
|
|
| SMILES |
CC1=CCC(C=C1)C(C)C
|
|
| InChI |
InChI=1S/C10H16/c1-8(2)10-6-4-9(3)5-7-10/h4-6,8,10H,7H2,1-3H3
|
|
| InChIKey |
OGLDWXZKYODSOB-UHFFFAOYSA-N
|
|
| Synonyms |
ALPHA-PHELLANDRENE; 99-83-2; p-Mentha-1,5-diene; Menthadiene; 1,3-Cyclohexadiene, 2-methyl-5-(1-methylethyl)-; alpha-Fellandrene; Dihydro-p-cymene; 5-Isopropyl-2-methyl-1,3-cyclohexadiene; .alpha.-Phellandrene; (-)-5-Isopropyl-2-methyl-1,3-cyclohexadiene; 4-Isopropyl-1-methyl-1,5-cyclohexadiene; 2-Methyl-5-isopropyl-1,3-cyclohexadiene; PHELLANDRENE; Phellandrene, alpha-; 2-methyl-5-propan-2-ylcyclohexa-1,3-diene; FEMA No. 2856; alpha Phellandrene; 1-Isopropyl-4-methyl-2,4-cyclohexadiene; 1-Methyl-4-isopropyl-1,5-cyclohexadiene; 5-isopropyl-2-methylcyclohexa-1,3-diene; 2-methyl-5-(1-methylethyl)-1,3-cyclohexadiene; (-)-2-Methyl-5-(1-methylethyl)-1,3-cyclohexadiene; alpha-Phellandrene, stabilized; 5-isopropyl-2-methyl-cyclohexa-1,3-diene; a-phellandrene; CHEBI:50035; NSC1842; 49JV13XE39; 1329-99-3; 2-methyl-5-(propan-2-yl)cyclohexa-1,3-diene; 1-phellandrene; alpha-Phellandrene (natural); HSDB 1130; EINECS 202-792-5; BRN 1280394; UNII-49JV13XE39; alpha-Phellandren; .alpha.-Fellandrene; 1,3-Cyclohexadiene, 2-methyl-5-(1-methylethyl)-, (R)-; PHELLANDRENE,ALPHA; .ALPHA. PHELLANDRENE; DSSTox_CID_27593; DSSTox_RID_82440; DSSTox_GSID_47593; 3-05-00-00342 (Beilstein Handbook Reference); ALPHA-PHELLANDRENE [FCC]; CHEMBL3188459; DTXSID4047593; FEMA 2856; FERRICORTHOPHOSPHATEHYDRATE; ALPHA-PHELLANDRENE [HSDB]; .RHO.-MENTHA-1,5-DIENE; .ALPHA.-PHELLANDRENE [MI]; AMY22322; NSC-1842; .ALPHA.-PHELLANDRENE [FHFI]; Tox21_302550; MFCD00040419; (+/-)-.ALPHA.-PHELLANDRENE; AKOS015913085; CAS-99-83-2; .ALPHA.-PHELLANDRENE, (+/-)-; 2-Methyl-5-isopropyl-1,3-cyclohexadien; NCGC00256667-01; AS-75606; alpha-Phellandrene, natural, >=85%, FG; DB-070271; FT-0607984; M0051; E77746; (+/-)-alpha-Phellandrene 100 microg/mL in Methanol; Q19606345
|
|
| CAS | 99-83-2 | |
| PubChem CID | 7460 | |
| ChEMBL ID | CHEMBL3188459 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 136.23 | ALogp: | 3.2 |
| HBD: | 0 | HBA: | 0 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 0.0 | Aromatic Rings: | 1 |
| Heavy Atoms: | 10 | QED Weighted: | 0.512 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.383 | MDCK Permeability: | 0.00002390 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.013 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.014 |
| 30% Bioavailability (F30%): | 0.146 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.835 | Plasma Protein Binding (PPB): | 92.01% |
| Volume Distribution (VD): | 2.963 | Fu: | 7.75% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.258 | CYP1A2-substrate: | 0.471 |
| CYP2C19-inhibitor: | 0.178 | CYP2C19-substrate: | 0.93 |
| CYP2C9-inhibitor: | 0.142 | CYP2C9-substrate: | 0.337 |
| CYP2D6-inhibitor: | 0.059 | CYP2D6-substrate: | 0.832 |
| CYP3A4-inhibitor: | 0.274 | CYP3A4-substrate: | 0.639 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 12.66 | Half-life (T1/2): | 0.617 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.024 | Human Hepatotoxicity (H-HT): | 0.76 |
| Drug-inuced Liver Injury (DILI): | 0.014 | AMES Toxicity: | 0.011 |
| Rat Oral Acute Toxicity: | 0.038 | Maximum Recommended Daily Dose: | 0.723 |
| Skin Sensitization: | 0.94 | Carcinogencity: | 0.344 |
| Eye Corrosion: | 0.183 | Eye Irritation: | 0.957 |
| Respiratory Toxicity: | 0.855 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000383 | 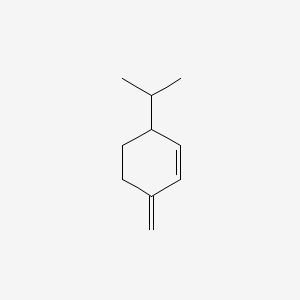 |
0.421 | D06GIP | 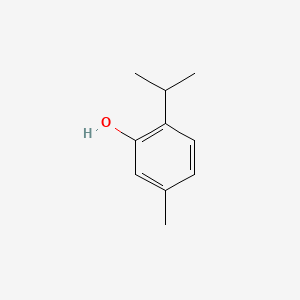 |
0.244 | ||
| ENC003463 | 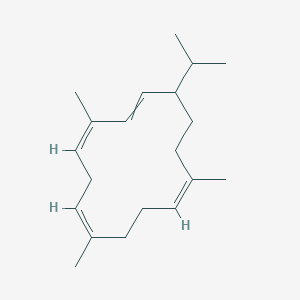 |
0.390 | D0A3HB | 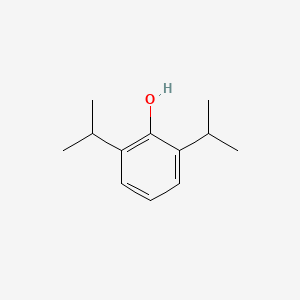 |
0.196 | ||
| ENC001809 | 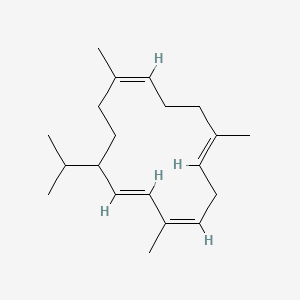 |
0.390 | D0X0RI | 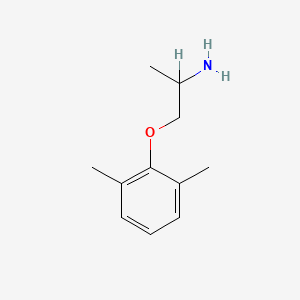 |
0.192 | ||
| ENC005117 | 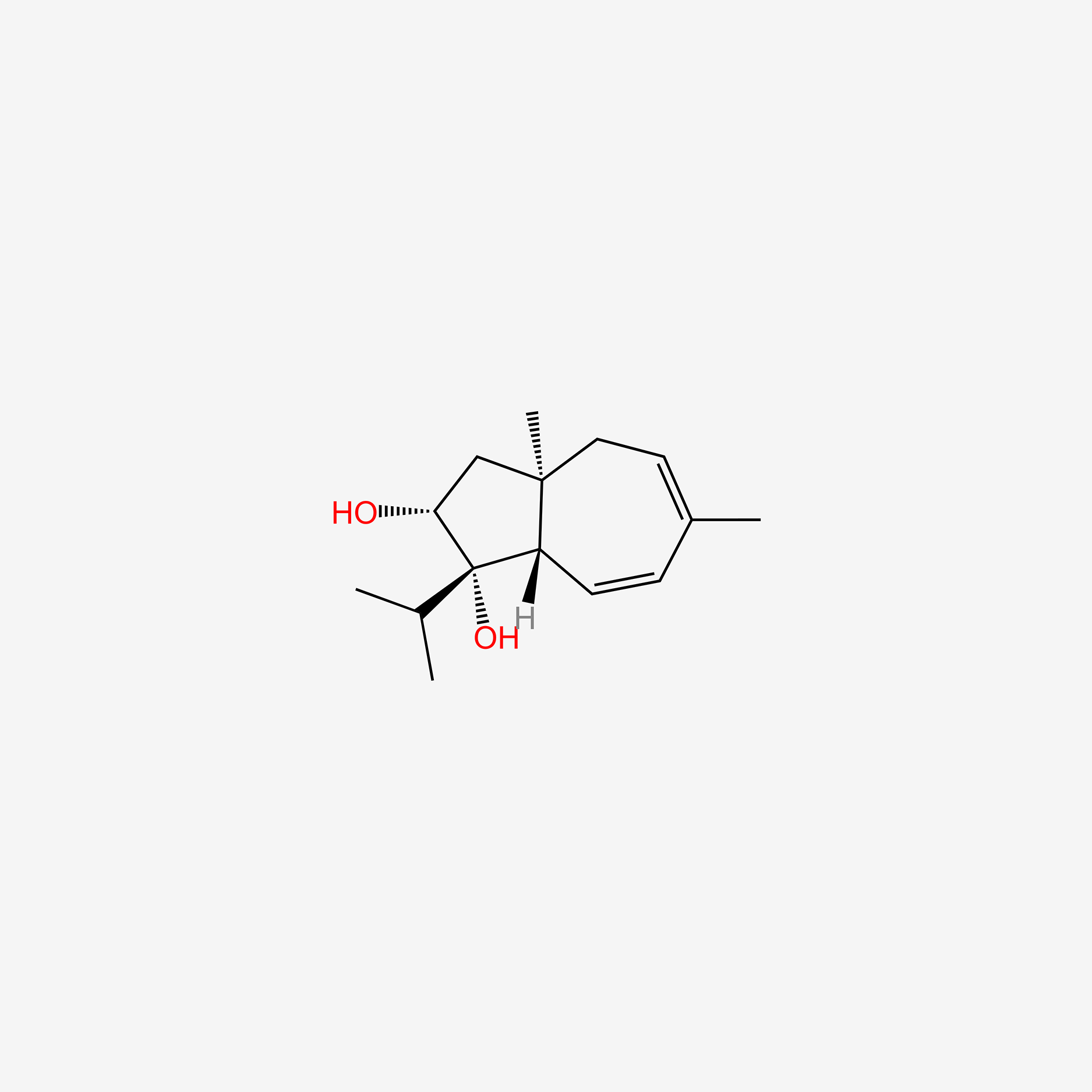 |
0.358 | D04CSZ | 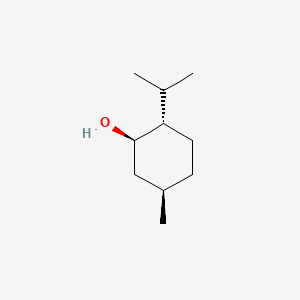 |
0.191 | ||
| ENC002264 | 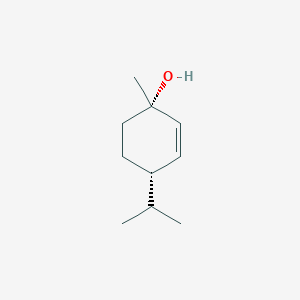 |
0.333 | D01PJR | 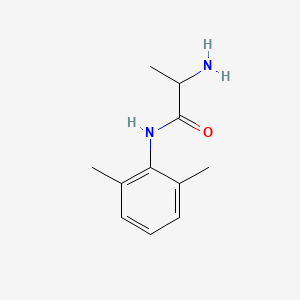 |
0.185 | ||
| ENC001837 | 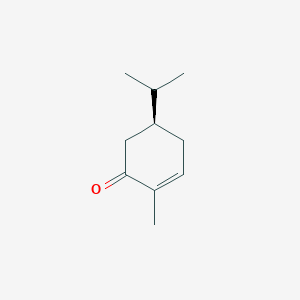 |
0.333 | D0YQ5L | 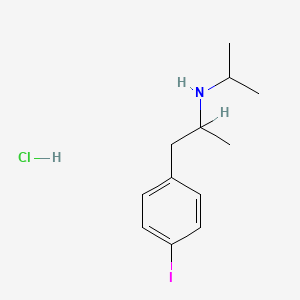 |
0.179 | ||
| ENC000872 | 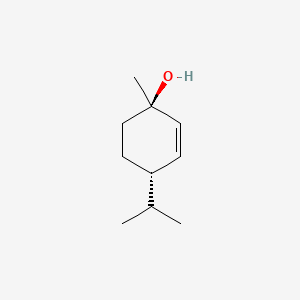 |
0.333 | D06IXT | 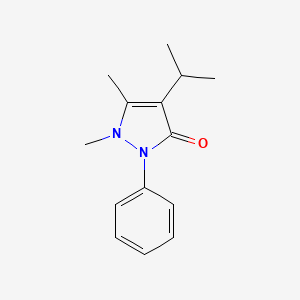 |
0.177 | ||
| ENC001308 | 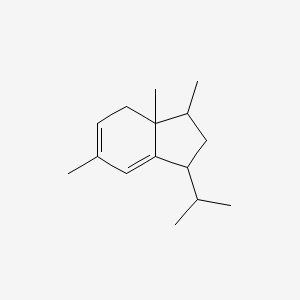 |
0.288 | D0R1QE | 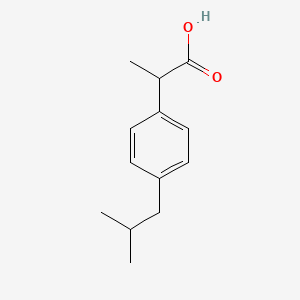 |
0.175 | ||
| ENC000197 | 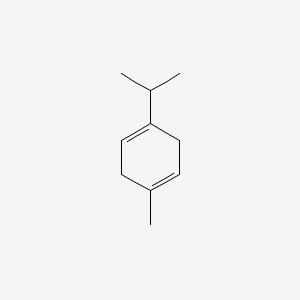 |
0.286 | D0U3DU | 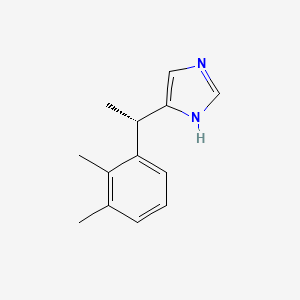 |
0.169 | ||
| ENC000520 | 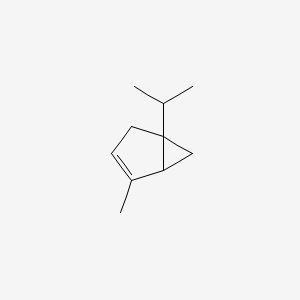 |
0.286 | D08KVZ | 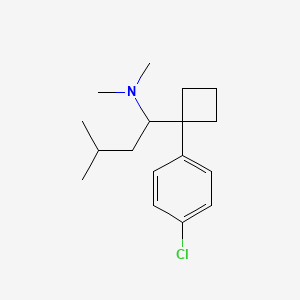 |
0.164 | ||