NPs Basic Information
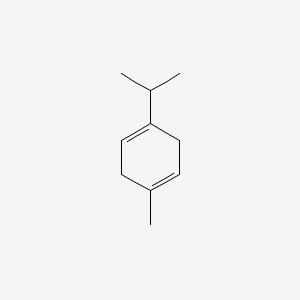
|
Name |
gamma-Terpinene
|
| Molecular Formula | C10H16 | |
| IUPAC Name* |
1-methyl-4-propan-2-ylcyclohexa-1,4-diene
|
|
| SMILES |
CC1=CCC(=CC1)C(C)C
|
|
| InChI |
InChI=1S/C10H16/c1-8(2)10-6-4-9(3)5-7-10/h4,7-8H,5-6H2,1-3H3
|
|
| InChIKey |
YKFLAYDHMOASIY-UHFFFAOYSA-N
|
|
| Synonyms |
GAMMA-TERPINENE; 99-85-4; p-Mentha-1,4-diene; Crithmene; Moslene; gamma-Terpinen; 1,4-p-Menthadiene; 4-Isopropyl-1-methyl-1,4-cyclohexadiene; .gamma.-Terpinen; .gamma.-Terpinene; 1,4-Cyclohexadiene, 1-methyl-4-(1-methylethyl)-; 1-methyl-4-propan-2-ylcyclohexa-1,4-diene; 1-Isopropyl-4-methyl-1,4-cyclohexadiene; TERPINENE, ALPHA; 1-Methyl-4-(1-methylethyl)-1,4-cyclohexadiene; FEMA No. 3559; 1-methyl-4-(propan-2-yl)cyclohexa-1,4-diene; 1-Methyl-4-isopropylcyclohexadiene-1,4; NSC 21448; 1-isopropyl-4-methylcyclohexa-1,4-diene; 1,4-Cyclohexadiene, 1-methyl-4-isopropyl-; 4YGF4PQP49; 1-Methyl-4-isopropyl-1,4-cyclohexadiene; CHEBI:10577; NSC-21448; Crithmene; Moslene; NSC 21448; gamma-Terpinene; Gamma-terpinene gamma-terpinene; gamma-Terpinene (natural); EINECS 202-794-6; UNII-4YGF4PQP49; Alpha terpinene; Gamma terpinene; AI3-26468; gamma -Terpinene; MFCD00001537; Terpinene, .gamma.-; gamma-Terpinene, 97%; 1-isopropyl-4-methyl-cyclohexa-1,4-diene; bmse000778; DSSTox_CID_21210; DSSTox_RID_79650; DSSTox_GSID_41210; 1, 1-methyl-4-isopropyl-; GAMMA-TERPINENE [FCC]; CHEMBL449693; DTXSID6041210; gamma-Terpinene, >=95%, FG; ZINC967594; NSC21448; gamma-Terpinene, analytical standard; Tox21_300963; WLN: L6U CUTJ AY1&1 D1; gamma-Terpinene, natural, 95%, FG; P-MENTHA-1,4-DIENE [FHFI]; AKOS015840812; HY-W020183; LMPR0102090027; CAS-99-85-4; NCGC00248232-01; NCGC00254865-01; BS-23574; gamma-Terpinene, purum, >=97.0% (GC); 1-Isopropyl-4-methyl-1,4-cyclohexadiene #; FT-0626623; M0318; C09900; E80754; EN300-211578; gamma-Terpinene 1000 microg/mL in Isopropanol; Q423975; W-100015; gamma-Terpinene, primary pharmaceutical reference standard; 9NI
|
|
| CAS | 99-85-4 | |
| PubChem CID | 7461 | |
| ChEMBL ID | CHEMBL449693 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 136.23 | ALogp: | 2.8 |
| HBD: | 0 | HBA: | 0 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 0.0 | Aromatic Rings: | 1 |
| Heavy Atoms: | 10 | QED Weighted: | 0.477 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.343 | MDCK Permeability: | 0.00002060 |
| Pgp-inhibitor: | 0.009 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.993 |
| 30% Bioavailability (F30%): | 0.996 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.386 | Plasma Protein Binding (PPB): | 93.74% |
| Volume Distribution (VD): | 4.876 | Fu: | 5.94% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.928 | CYP1A2-substrate: | 0.243 |
| CYP2C19-inhibitor: | 0.34 | CYP2C19-substrate: | 0.769 |
| CYP2C9-inhibitor: | 0.313 | CYP2C9-substrate: | 0.834 |
| CYP2D6-inhibitor: | 0.11 | CYP2D6-substrate: | 0.432 |
| CYP3A4-inhibitor: | 0.123 | CYP3A4-substrate: | 0.228 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 12.063 | Half-life (T1/2): | 0.409 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.003 | Human Hepatotoxicity (H-HT): | 0.117 |
| Drug-inuced Liver Injury (DILI): | 0.523 | AMES Toxicity: | 0.008 |
| Rat Oral Acute Toxicity: | 0.021 | Maximum Recommended Daily Dose: | 0.042 |
| Skin Sensitization: | 0.077 | Carcinogencity: | 0.961 |
| Eye Corrosion: | 0.245 | Eye Irritation: | 0.951 |
| Respiratory Toxicity: | 0.029 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC003075 | 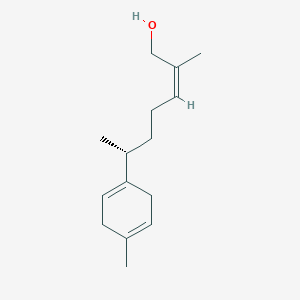 |
0.479 | D06GIP | 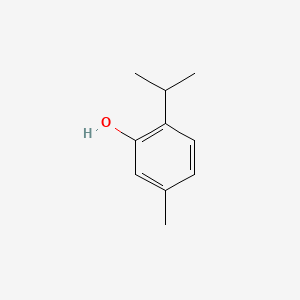 |
0.273 | ||
| ENC000198 | 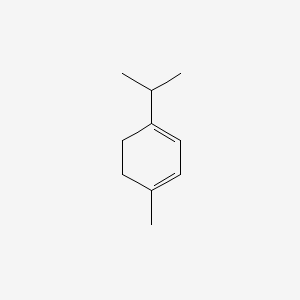 |
0.459 | D0A3HB | 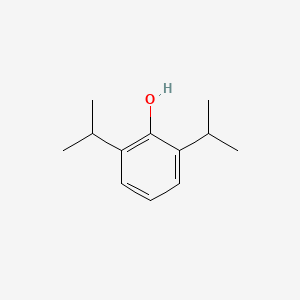 |
0.220 | ||
| ENC001824 | 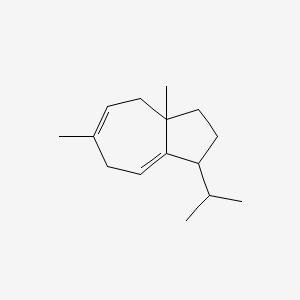 |
0.388 | D06IXT | 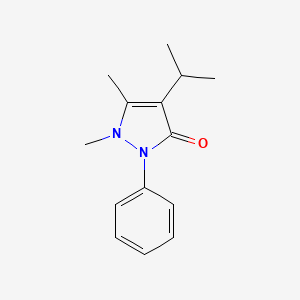 |
0.197 | ||
| ENC003087 | 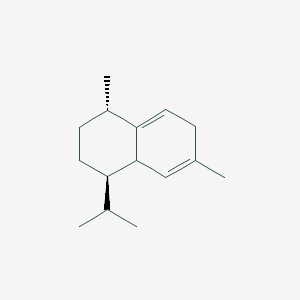 |
0.333 | D0X0RI |  |
0.192 | ||
| ENC000395 |  |
0.317 | D01PJR |  |
0.185 | ||
| ENC005518 |  |
0.306 | D08KVZ | 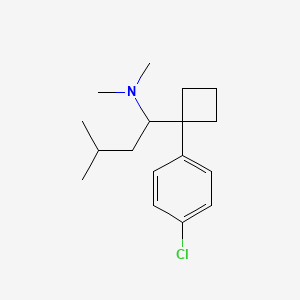 |
0.182 | ||
| ENC000165 | 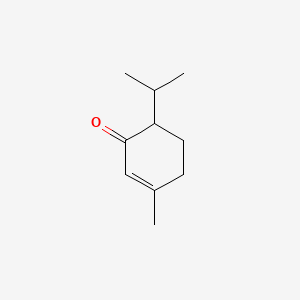 |
0.302 | D0YQ5L | 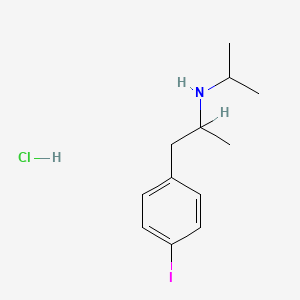 |
0.179 | ||
| ENC000388 | 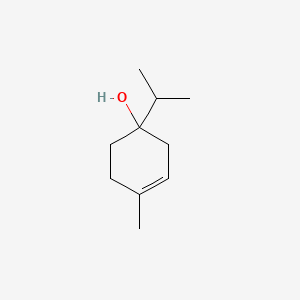 |
0.302 | D03QJL | 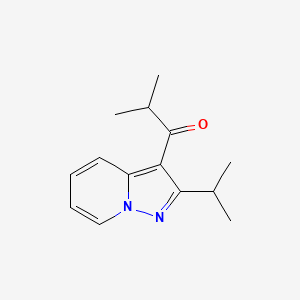 |
0.177 | ||
| ENC001837 | 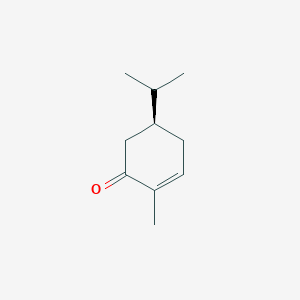 |
0.302 | D0R1QE | 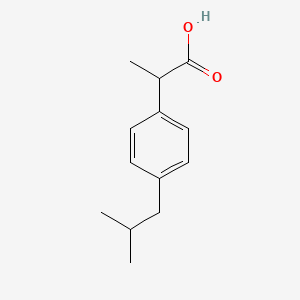 |
0.175 | ||
| ENC001637 | 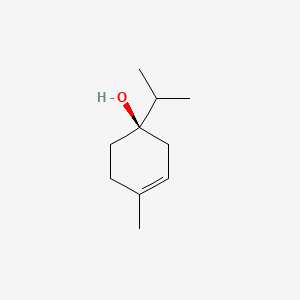 |
0.302 | D0U3DU | 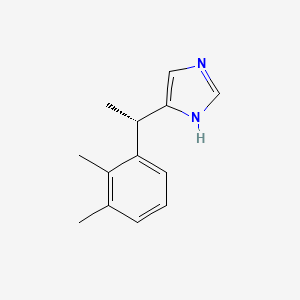 |
0.169 | ||