NPs Basic Information
|
Name |
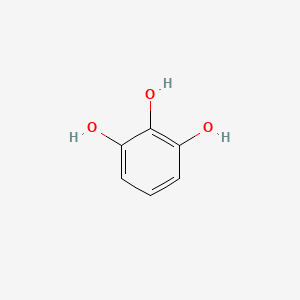
Pyrogallol
|
| Molecular Formula | C6H6O3 | |
| IUPAC Name* |
benzene-1,2,3-triol
|
|
| SMILES |
C1=CC(=C(C(=C1)O)O)O
|
|
| InChI |
InChI=1S/C6H6O3/c7-4-2-1-3-5(8)6(4)9/h1-3,7-9H
|
|
| InChIKey |
WQGWDDDVZFFDIG-UHFFFAOYSA-N
|
|
| Synonyms |
pyrogallol; benzene-1,2,3-triol; 87-66-1; 1,2,3-trihydroxybenzene; pyrogallic acid; 1,2,3-benzenetriol; fourrine PG; Fouramine Brown AP; fourrine 85; Pyro; Piral; C.I. Oxidation Base 32; Benzenetriol; fouramine base ap; C.I. 76515; 1,2,3-Trihydroxybenzen; Benzene, 1,2,3-trihydroxy-; NSC 5035; 2,3-Dihydroxyphenol; 1,2,3-Trihydroxybenzen [Czech]; 1,2,3-TRIHYDROXY-BENZENE; NSC-5035; 01Y4A2QXY0; CHEMBL307145; 35296-77-6; CHEBI:16164; Benzene-1,2,3-triol (Pyrogallol); MFCD00002192; NCGC00091507-01; 1, 2, 3-Benzenetriol; DSSTox_CID_5983; DSSTox_RID_77980; DSSTox_GSID_25983; 30813-84-4; 1,2,3-Trihydroxybenzen (CZECH); CI Oxidation Base 32; CAS-87-66-1; Pyrogallol [NF]; PYG; CCRIS 1940; HSDB 794; PYROGALLOL, ACS; EINECS 201-762-9; BRN 0907431; UNII-01Y4A2QXY0; CI 76515; Pyrogallol;; trihydroxybenzene; AI3-00709; Pyrogallol-[d6]; 1,3-Benzenetriol; Pyrogallol, 98%; PYROP; Pyrogallol ACS grade; 1,3-Trihydroxybenzen; 1216684-97-7; Pyrogallic Acid,(S); 1,3-Trihydroxybenzene; benzene-1,2-3-triol; PYROGALLOL [MI]; Pyrogallol, ACS reagent; PYROGALLOL [HSDB]; PYROGALLOL [INCI]; Benzene,2,3-trihydroxy-; WLN: QR BQ CQ; PYROGALLOL [VANDF]; PYROGALLOL [MART.]; SCHEMBL3532; PYROGALLOL [WHO-DD]; C.I. Oxidation Base 32; 4-06-00-07327 (Beilstein Handbook Reference); MLS001066376; Pyrogallol, analytical standard; 1,2,3-Trihydroxybenzene, XIV; DTXSID6025983; Pyrogallol, >=98% (HPLC); Pyrogallol, p.a., ACS reagent; NSC5035; ZINC330141; BCP15871; HY-N1579; Pyrogallol, ACS reagent, >=99%; STR08708; Tox21_111143; Tox21_202373; BBL011607; BDBM50031472; Pyrogallol, Vetec(TM) reagent grade; s3885; STL163335; AKOS000120163; AM10660; CCG-266100; CS-W019928; 1,2,3-Benzenetriol (ACD/Name 4.0); NCGC00091507-02; NCGC00091507-03; NCGC00259922-01; Pyrogallol, purum, >=98.0% (HPLC); AC-11384; BP-12538; DA-40956; GMN; Pyrogallol, SAJ first grade, >=98.0%; SMR000471842; Pyrogallol, JIS special grade, >=99.0%; FT-0606230; P0570; EN300-18055; C01108; AB-131/40221933; Q388692; W-104009; Z57127553; 2,3-Dihydroxyphenol; Benzene-1,2,3-triol; NSC 5035; F0001-2163
|
|
| CAS | 87-66-1 | |
| PubChem CID | 1057 | |
| ChEMBL ID | CHEMBL307145 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 126.11 | ALogp: | 0.5 |
| HBD: | 3 | HBA: | 3 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 60.7 | Aromatic Rings: | 1 |
| Heavy Atoms: | 9 | QED Weighted: | 0.458 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.895 | MDCK Permeability: | 0.00001400 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.046 | 20% Bioavailability (F20%): | 0.982 |
| 30% Bioavailability (F30%): | 0.959 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.043 | Plasma Protein Binding (PPB): | 71.10% |
| Volume Distribution (VD): | 0.307 | Fu: | 21.64% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.102 | CYP1A2-substrate: | 0.305 |
| CYP2C19-inhibitor: | 0.054 | CYP2C19-substrate: | 0.061 |
| CYP2C9-inhibitor: | 0.1 | CYP2C9-substrate: | 0.627 |
| CYP2D6-inhibitor: | 0.08 | CYP2D6-substrate: | 0.374 |
| CYP3A4-inhibitor: | 0.04 | CYP3A4-substrate: | 0.133 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 19.339 | Half-life (T1/2): | 0.935 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.01 | Human Hepatotoxicity (H-HT): | 0.051 |
| Drug-inuced Liver Injury (DILI): | 0.06 | AMES Toxicity: | 0.7 |
| Rat Oral Acute Toxicity: | 0.559 | Maximum Recommended Daily Dose: | 0.028 |
| Skin Sensitization: | 0.948 | Carcinogencity: | 0.638 |
| Eye Corrosion: | 0.974 | Eye Irritation: | 0.975 |
| Respiratory Toxicity: | 0.84 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000404 | 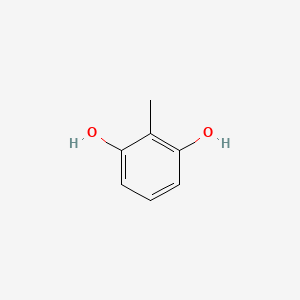 |
0.600 | D07MOX | 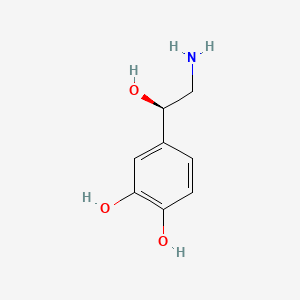 |
0.366 | ||
| ENC000021 | 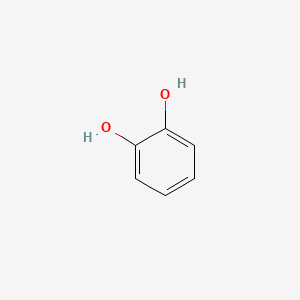 |
0.533 | D0T7OW | 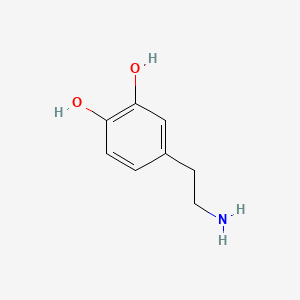 |
0.350 | ||
| ENC000690 | 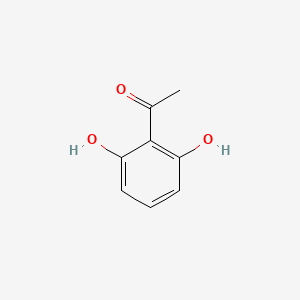 |
0.514 | D07HBX |  |
0.342 | ||
| ENC002350 | 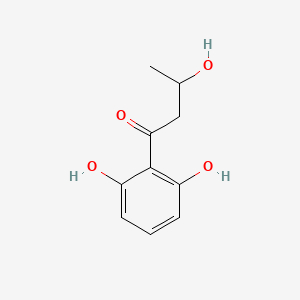 |
0.452 | D0V9EN |  |
0.341 | ||
| ENC000683 | 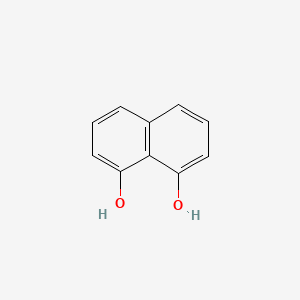 |
0.450 | D04PHC | 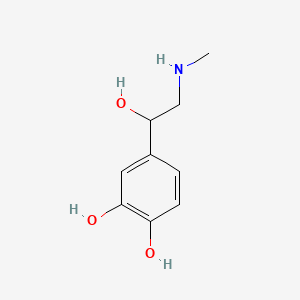 |
0.341 | ||
| ENC001513 | 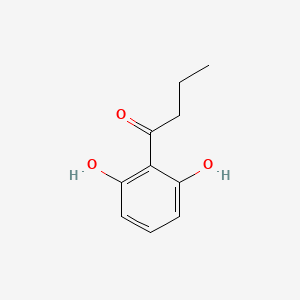 |
0.439 | D07EXH | 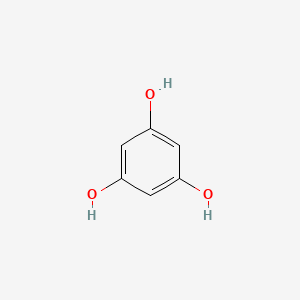 |
0.333 | ||
| ENC000329 | 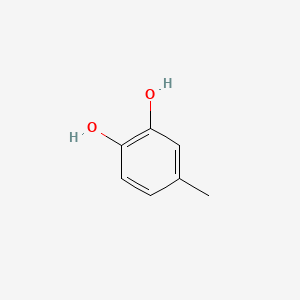 |
0.412 | D08HVR | 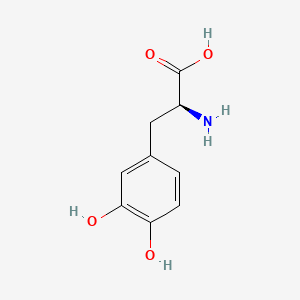 |
0.326 | ||
| ENC002237 | 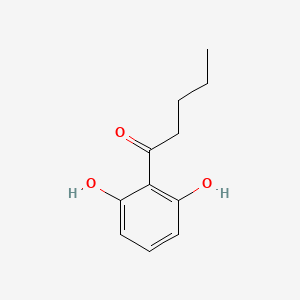 |
0.409 | D03UOT |  |
0.314 | ||
| ENC000390 | 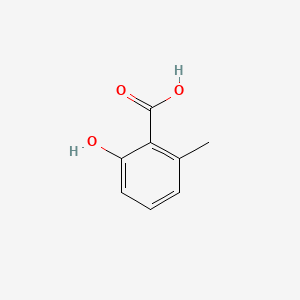 |
0.395 | D0I3RO | 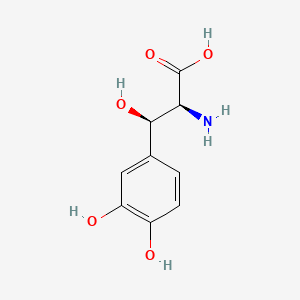 |
0.313 | ||
| ENC000002 | 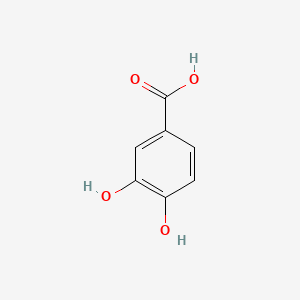 |
0.395 | D0BA6T | 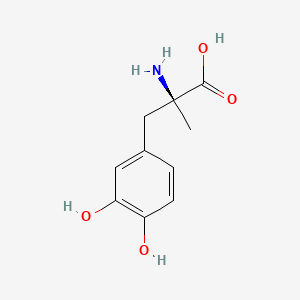 |
0.313 | ||