NPs Basic Information
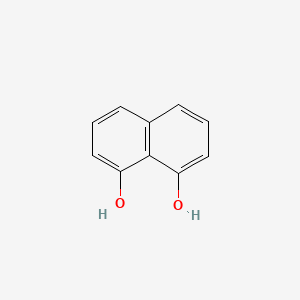
|
Name |
Naphthalene-1,8-diol
|
| Molecular Formula | C10H8O2 | |
| IUPAC Name* |
naphthalene-1,8-diol
|
|
| SMILES |
C1=CC2=C(C(=C1)O)C(=CC=C2)O
|
|
| InChI |
InChI=1S/C10H8O2/c11-8-5-1-3-7-4-2-6-9(12)10(7)8/h1-6,11-12H
|
|
| InChIKey |
OENHRRVNRZBNNS-UHFFFAOYSA-N
|
|
| Synonyms |
Naphthalene-1,8-diol; 1,8-Naphthalenediol; 569-42-6; 1,8-Dihydroxynaphthalene; JEW2VB8R33; MFCD00042701; 138999-35-6; 1,8-Naphthalenediol, radical ion(1+); EINECS 209-316-5; 1,8-Naphthalindiol; 8-hydroxy-1-naphthol; 1-Hydroxy-8-naphthol; UNII-JEW2VB8R33; SCHEMBL70219; CHEMBL203537; YSSJ3004; 1,8-Dihydroxynaphthalene, 95%; 1,8-DHN; DTXSID80205435; CHEBI:167604; BCP10859; CS-B0783; ZINC1846526; AC-950; AKOS003601449; AB90058; FS-3227; SY027091; A8134; AM20050054; FT-0607047; EN300-41347; Z415933894
|
|
| CAS | 138999-35-6 | |
| PubChem CID | 68438 | |
| ChEMBL ID | CHEMBL203537 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 160.17 | ALogp: | 2.8 |
| HBD: | 2 | HBA: | 2 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 40.5 | Aromatic Rings: | 2 |
| Heavy Atoms: | 12 | QED Weighted: | 0.622 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.629 | MDCK Permeability: | 0.00001840 |
| Pgp-inhibitor: | 0.003 | Pgp-substrate: | 0.003 |
| Human Intestinal Absorption (HIA): | 0.093 | 20% Bioavailability (F20%): | 0.975 |
| 30% Bioavailability (F30%): | 0.989 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.284 | Plasma Protein Binding (PPB): | 95.31% |
| Volume Distribution (VD): | 0.669 | Fu: | 3.54% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.987 | CYP1A2-substrate: | 0.631 |
| CYP2C19-inhibitor: | 0.454 | CYP2C19-substrate: | 0.09 |
| CYP2C9-inhibitor: | 0.518 | CYP2C9-substrate: | 0.895 |
| CYP2D6-inhibitor: | 0.812 | CYP2D6-substrate: | 0.857 |
| CYP3A4-inhibitor: | 0.416 | CYP3A4-substrate: | 0.194 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 13.317 | Half-life (T1/2): | 0.819 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.011 | Human Hepatotoxicity (H-HT): | 0.017 |
| Drug-inuced Liver Injury (DILI): | 0.248 | AMES Toxicity: | 0.668 |
| Rat Oral Acute Toxicity: | 0.624 | Maximum Recommended Daily Dose: | 0.039 |
| Skin Sensitization: | 0.943 | Carcinogencity: | 0.84 |
| Eye Corrosion: | 0.799 | Eye Irritation: | 0.994 |
| Respiratory Toxicity: | 0.915 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC002077 | 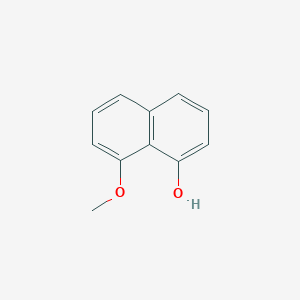 |
0.651 | D03UOT |  |
0.333 | ||
| ENC003034 | 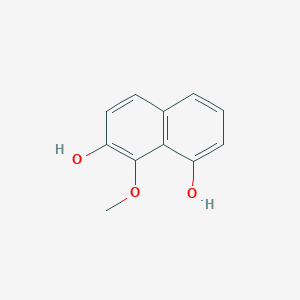 |
0.587 | D07HBX |  |
0.326 | ||
| ENC000021 | 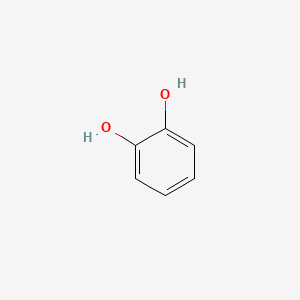 |
0.474 | D0O6IZ | 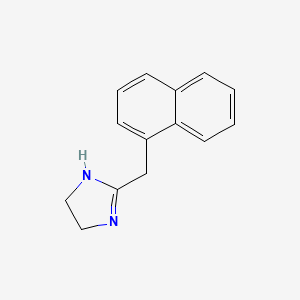 |
0.323 | ||
| ENC001512 | 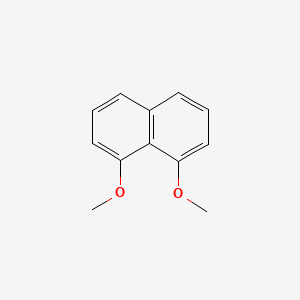 |
0.451 | D0Y0JH | 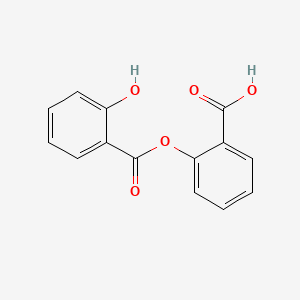 |
0.318 | ||
| ENC000404 | 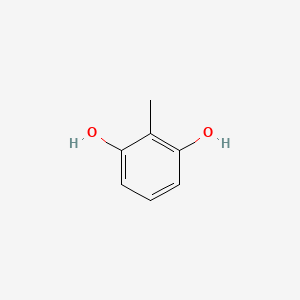 |
0.450 | D0L5PO | 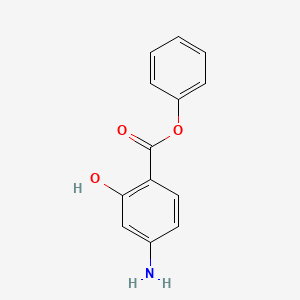 |
0.302 | ||
| ENC000060 | 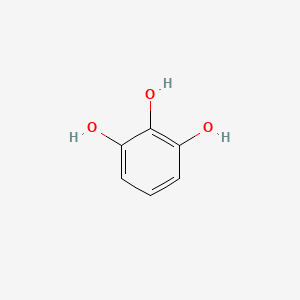 |
0.450 | D04JEE | 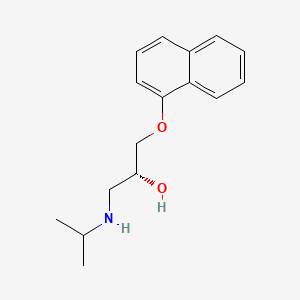 |
0.294 | ||
| ENC000087 | 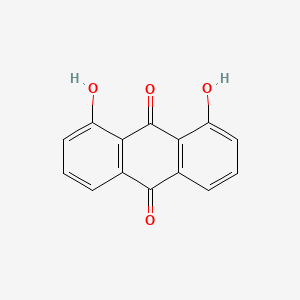 |
0.448 | D0F5ZM | 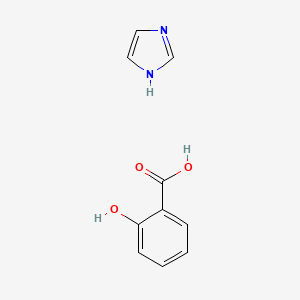 |
0.288 | ||
| ENC004888 | 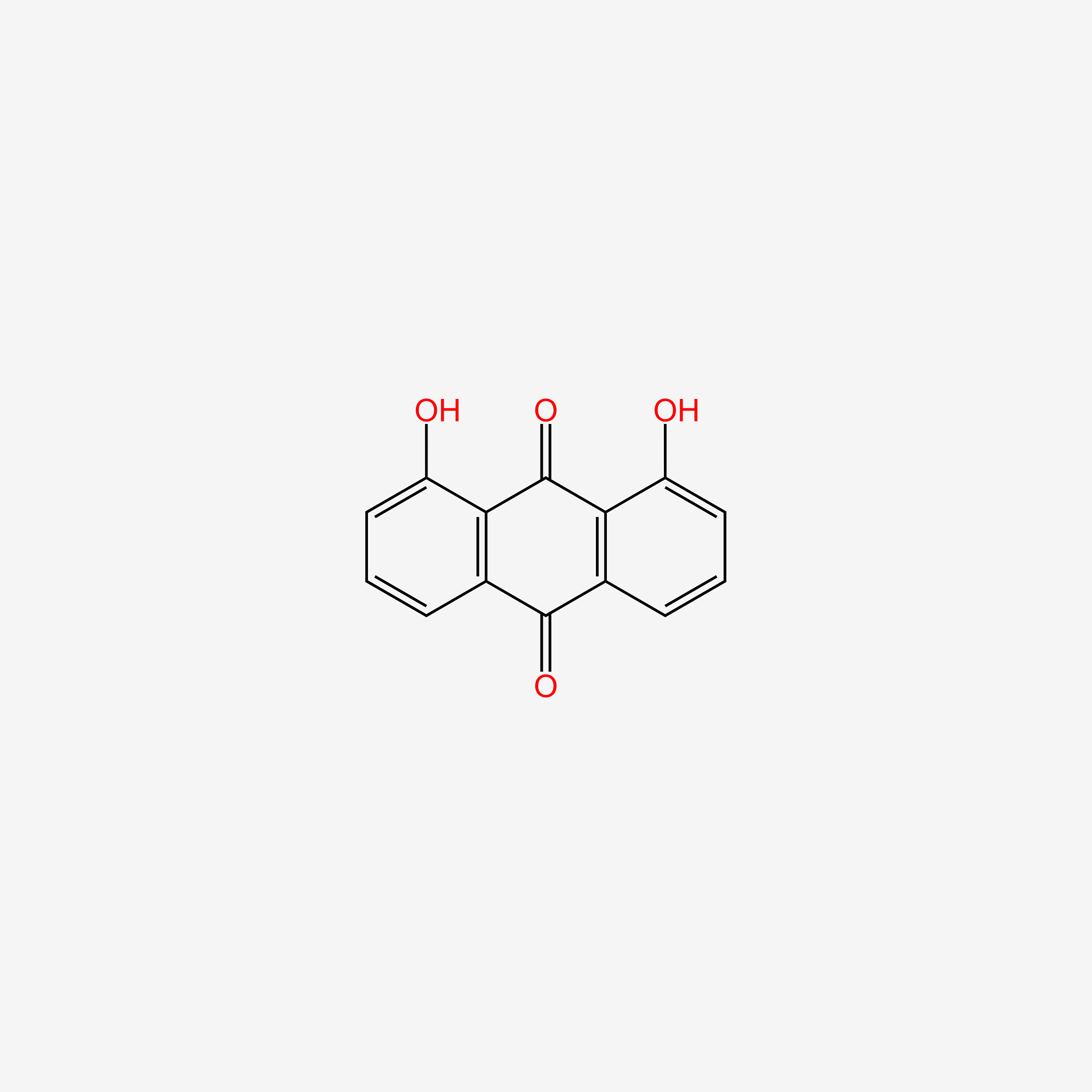 |
0.448 | D0H6QU | 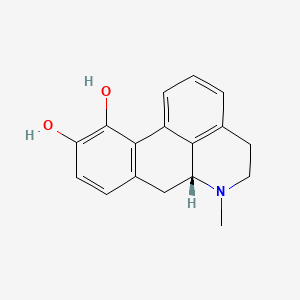 |
0.282 | ||
| ENC004820 | 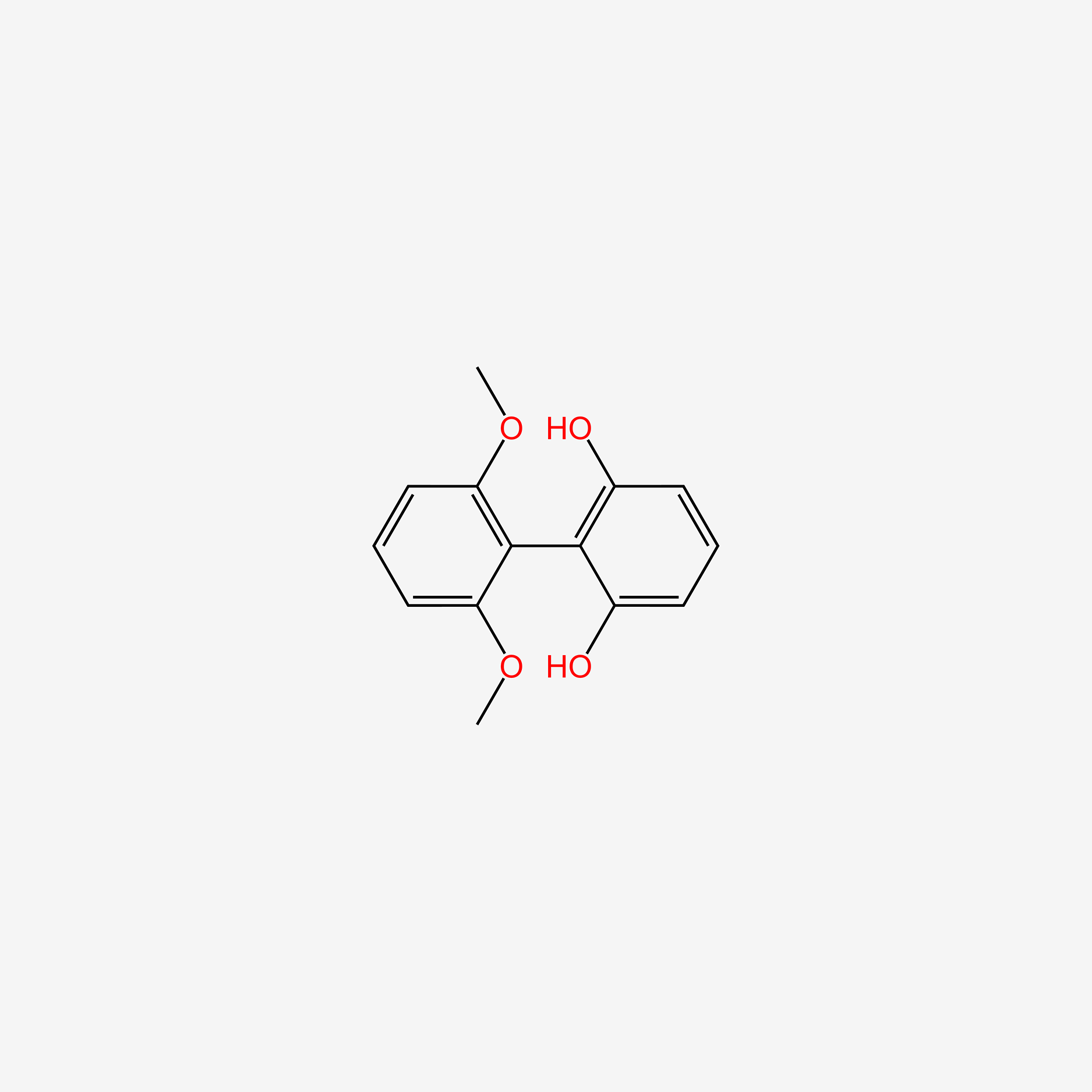 |
0.448 | D0T7OW | 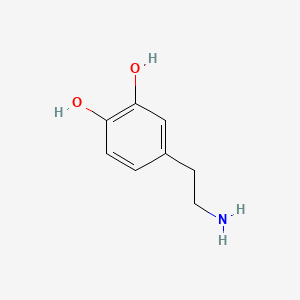 |
0.280 | ||
| ENC003644 | 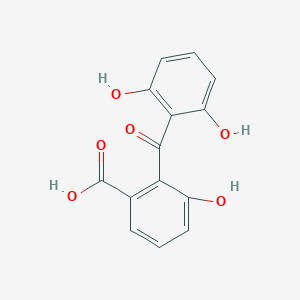 |
0.419 | D0H6TP | 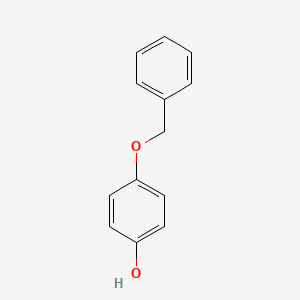 |
0.279 | ||