NPs Basic Information
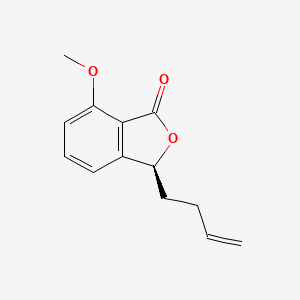
|
Name |
Asperetide
|
| Molecular Formula | C13H14O3 | |
| IUPAC Name* |
(3S)-3-but-3-enyl-7-methoxy-3H-2-benzofuran-1-one
|
|
| SMILES |
COC1=CC=CC2=C1C(=O)O[C@H]2CCC=C
|
|
| InChI |
InChI=1S/C13H14O3/c1-3-4-7-10-9-6-5-8-11(15-2)12(9)13(14)16-10/h3,5-6,8,10H,1,4,7H2,2H3/t10-/m0/s1
|
|
| InChIKey |
PZUFYUXNVZQDTO-JTQLQIEISA-N
|
|
| Synonyms |
Asperetide
|
|
| CAS | NA | |
| PubChem CID | 146684063 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 218.25 | ALogp: | 2.8 |
| HBD: | 0 | HBA: | 3 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 35.5 | Aromatic Rings: | 2 |
| Heavy Atoms: | 16 | QED Weighted: | 0.571 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.591 | MDCK Permeability: | 0.00002610 |
| Pgp-inhibitor: | 0.201 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.003 |
| 30% Bioavailability (F30%): | 0.066 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.384 | Plasma Protein Binding (PPB): | 83.71% |
| Volume Distribution (VD): | 1.088 | Fu: | 11.33% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.877 | CYP1A2-substrate: | 0.947 |
| CYP2C19-inhibitor: | 0.879 | CYP2C19-substrate: | 0.816 |
| CYP2C9-inhibitor: | 0.499 | CYP2C9-substrate: | 0.93 |
| CYP2D6-inhibitor: | 0.129 | CYP2D6-substrate: | 0.912 |
| CYP3A4-inhibitor: | 0.788 | CYP3A4-substrate: | 0.313 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 10 | Half-life (T1/2): | 0.189 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.019 | Human Hepatotoxicity (H-HT): | 0.095 |
| Drug-inuced Liver Injury (DILI): | 0.223 | AMES Toxicity: | 0.829 |
| Rat Oral Acute Toxicity: | 0.464 | Maximum Recommended Daily Dose: | 0.191 |
| Skin Sensitization: | 0.818 | Carcinogencity: | 0.8 |
| Eye Corrosion: | 0.675 | Eye Irritation: | 0.98 |
| Respiratory Toxicity: | 0.804 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC003052 | 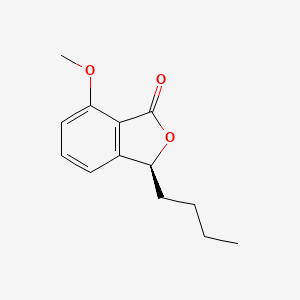 |
0.698 | D0E9CD |  |
0.271 | ||
| ENC001451 | 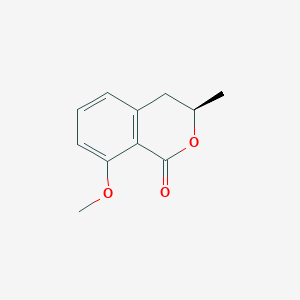 |
0.474 | D0L1WV | 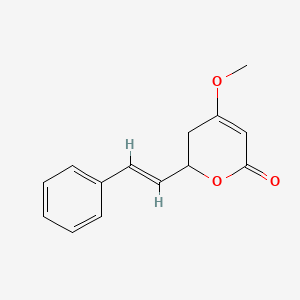 |
0.253 | ||
| ENC005942 | 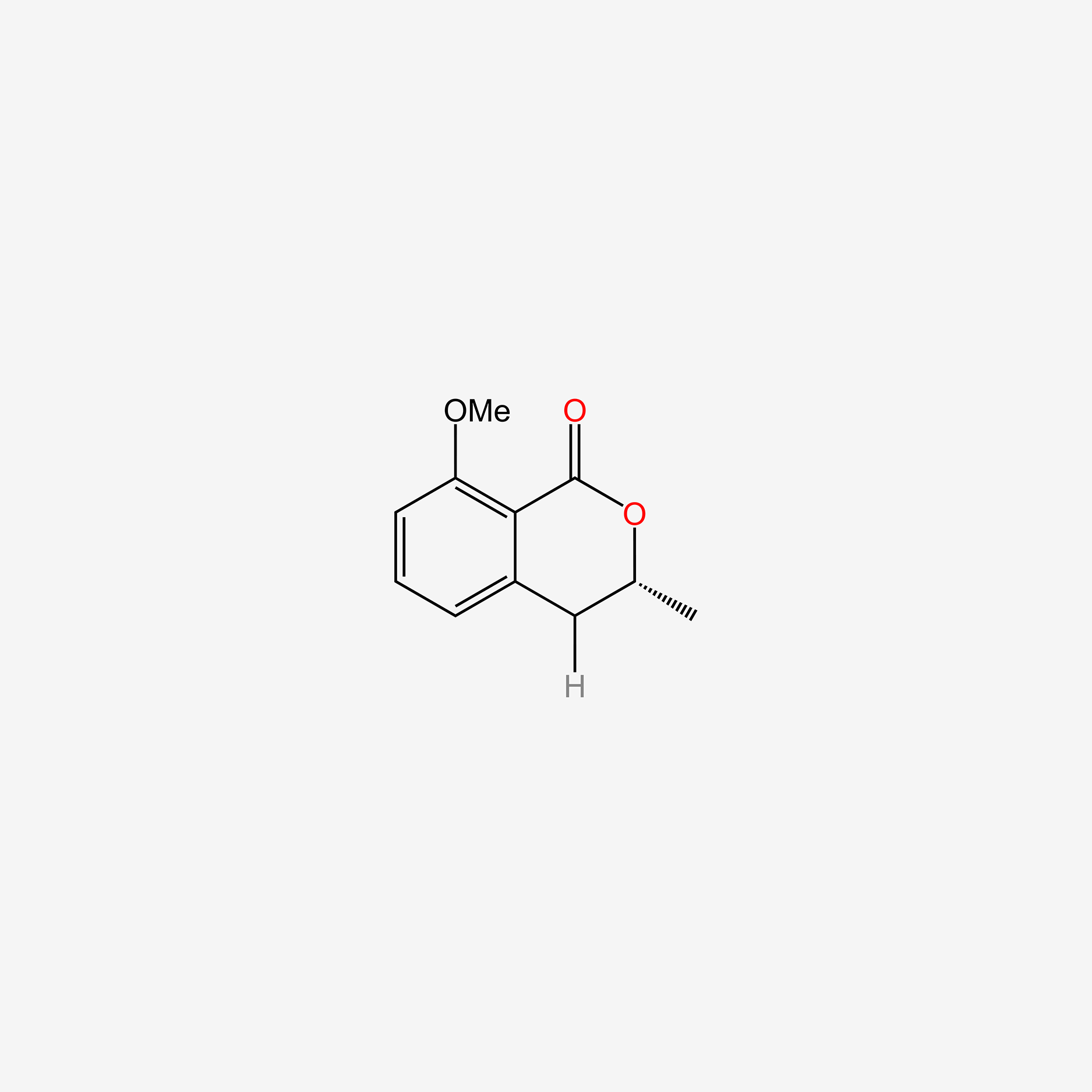 |
0.474 | D08CCE | 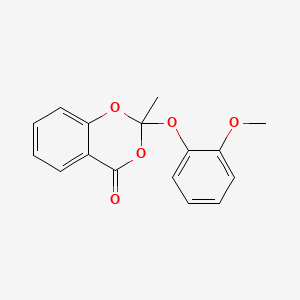 |
0.250 | ||
| ENC005578 | 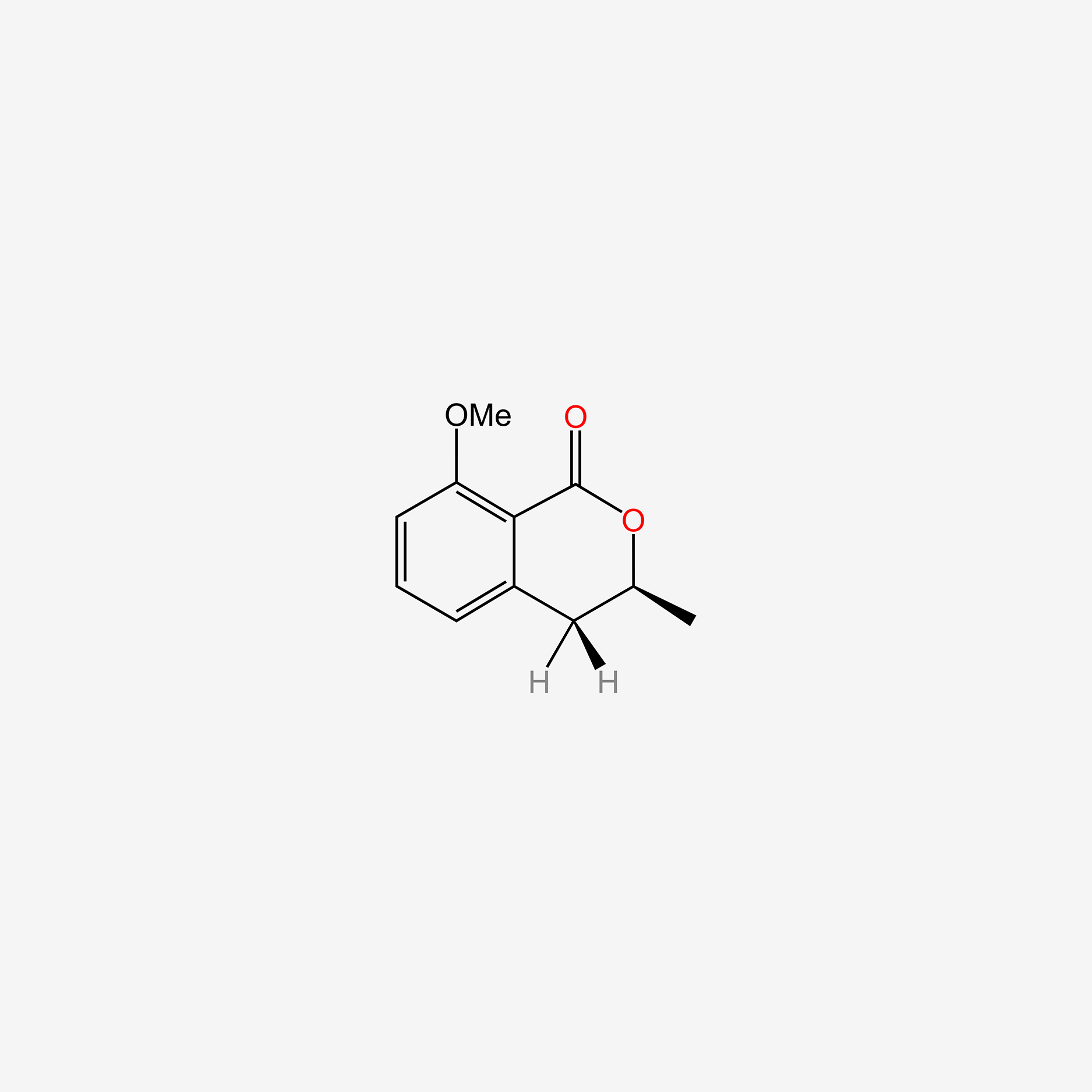 |
0.474 | D07MGA |  |
0.247 | ||
| ENC004821 | 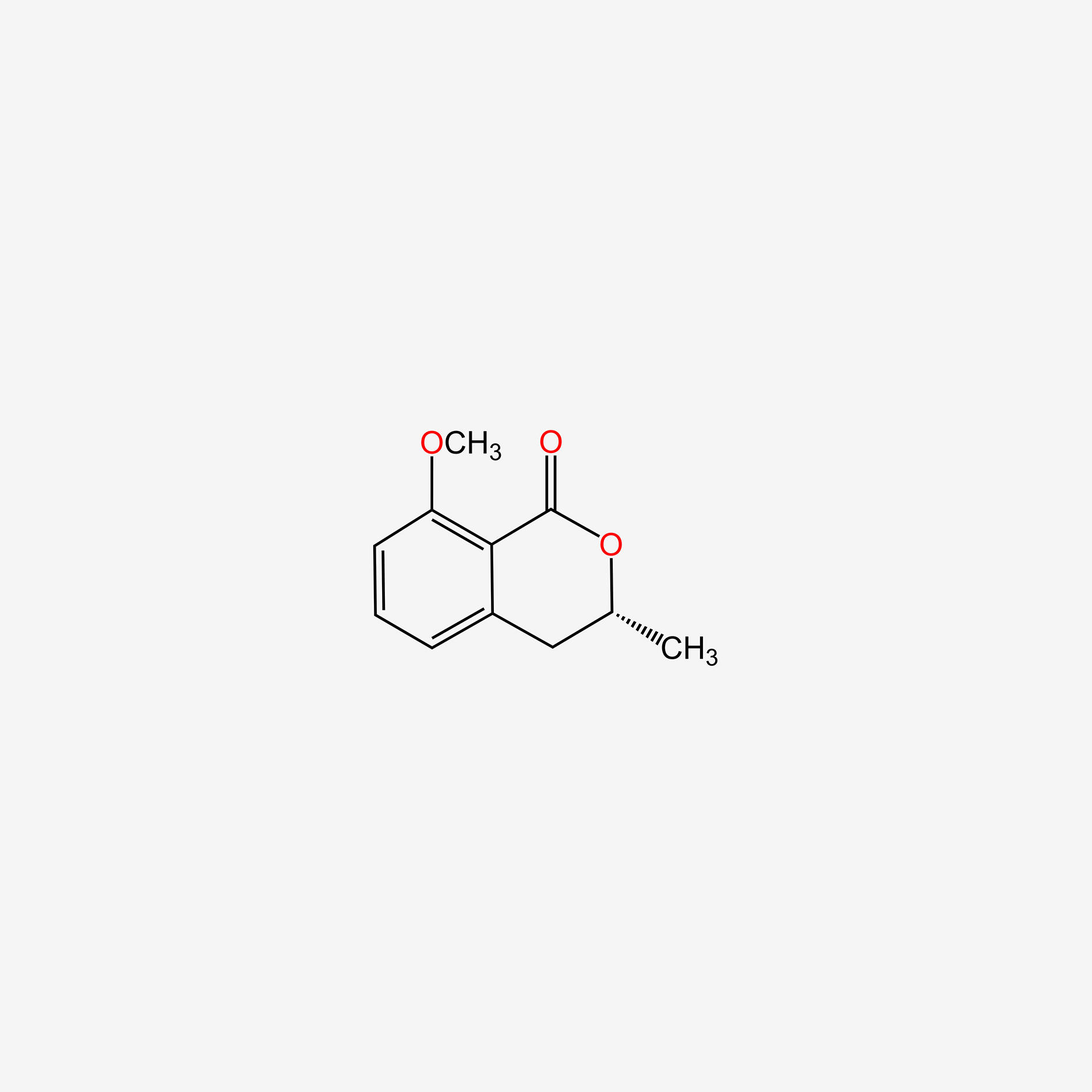 |
0.474 | D0C6OQ | 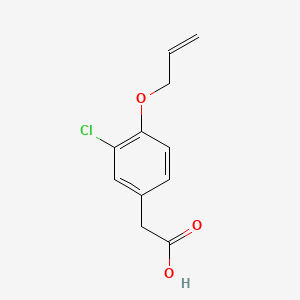 |
0.246 | ||
| ENC005190 | 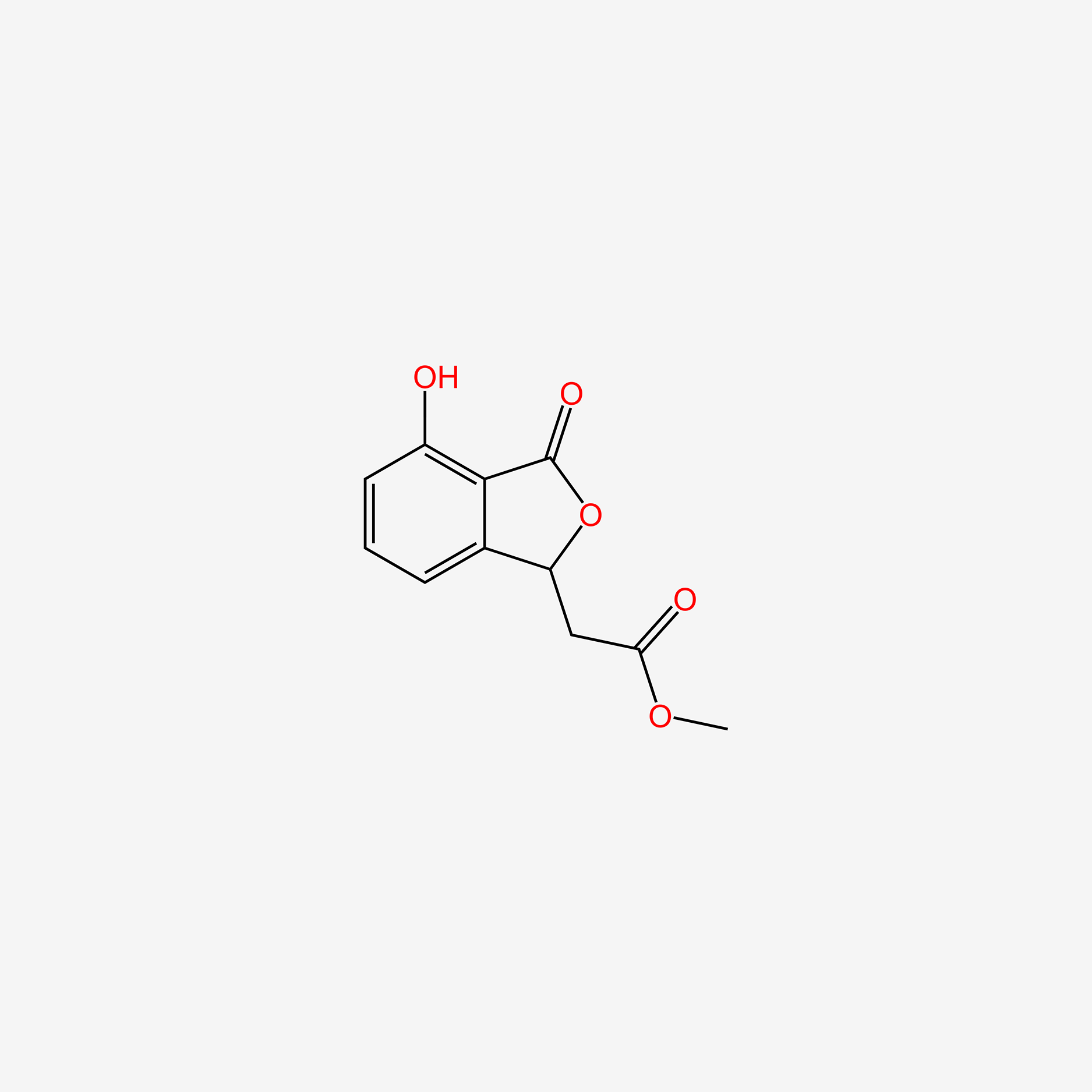 |
0.459 | D0FN7J | 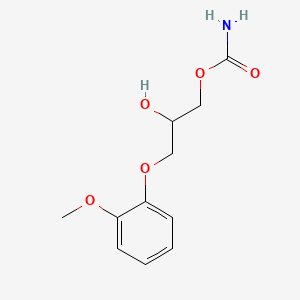 |
0.243 | ||
| ENC002458 | 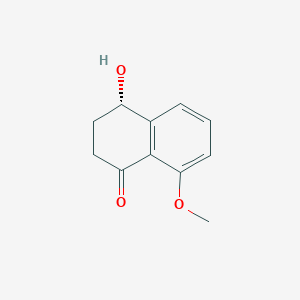 |
0.424 | D0L1JW |  |
0.238 | ||
| ENC002342 | 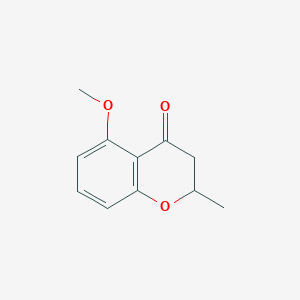 |
0.424 | D0Q5MQ | 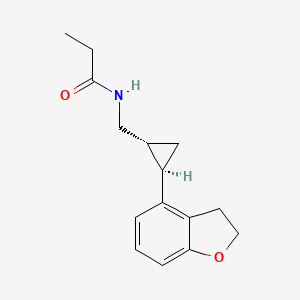 |
0.228 | ||
| ENC002190 | 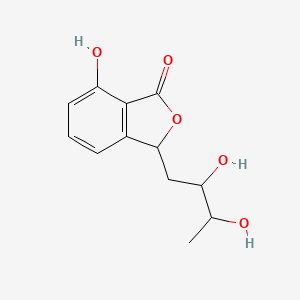 |
0.400 | D04TDQ | 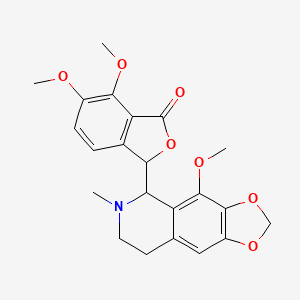 |
0.226 | ||
| ENC001305 | 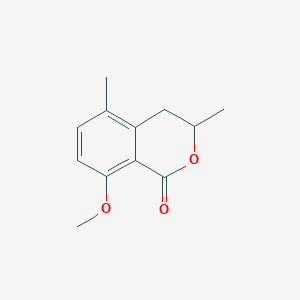 |
0.365 | D03GCJ | 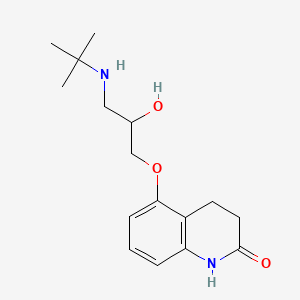 |
0.226 | ||