NPs Basic Information
|
Name |
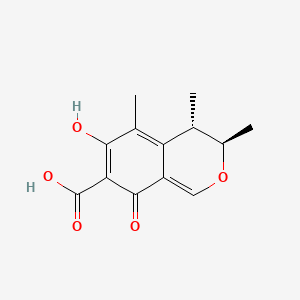
Citrinin
|
| Molecular Formula | C13H14O5 | |
| IUPAC Name* |
(3R,4S)-6-hydroxy-3,4,5-trimethyl-8-oxo-3,4-dihydroisochromene-7-carboxylic acid
|
|
| SMILES |
C[C@@H]1[C@H](OC=C2C1=C(C(=C(C2=O)C(=O)O)O)C)C
|
|
| InChI |
InChI=1S/C13H14O5/c1-5-7(3)18-4-8-9(5)6(2)11(14)10(12(8)15)13(16)17/h4-5,7,14H,1-3H3,(H,16,17)/t5-,7-/m1/s1
|
|
| InChIKey |
CBGDIJWINPWWJW-IYSWYEEDSA-N
|
|
| Synonyms |
citrinin; 518-75-2; (-)-citrinin; Citriain; (3R,4S)-4,6-Dihydro-8-hydroxy-3,4,5-trimethyl-6-oxo-3H-2-benzopyran-7-carboxylic acid; 4,6-Dihydro-8-hydroxy-3,4,5-trimethyl-6-oxo-3H-2-benzopyran-7-carboxylic acid; NSC186; 3H-2-Benzopyran-7-carboxylic acid, 4,6-dihydro-8-hydroxy-3,4,5-trimethyl-6-oxo-, (3R-trans)-; 3S697X6SNZ; CHEBI:48707; NSC-186; (3R,4S)-6-hydroxy-3,4,5-trimethyl-8-oxo-3,4-dihydroisochromene-7-carboxylic acid; (3R,4S)-8-hydroxy-3,4,5-trimethyl-6-oxo-4,6-dihydro-3H-isochromene-7-carboxylic acid; (3R-trans)-4,6-dihydro-8-hydroxy-3,4,5-trimethyl-6-oxo-3H-2-benzopyran-7-carboxylic acid; Citrinin 100 microg/mL in Acetonitrile; 3H-2-Benzopyran-7-carboxylic acid, 4,6-dihydro-8-hydroxy-3,4,5-trimethyl-6-oxo-, (3R,4S)-; (3R,4S)-8-hydroxy-3,4,5-trimethyl-6-oxo-3,4-dihydroisochromene-7-carboxylic acid; CCRIS 175; HSDB 3473; EINECS 208-257-2; BRN 0088597; UNII-3S697X6SNZ; 3,4-Dihydro-8-hydroxy-3,4,5-trimethyl-6H-6-oxobenzo(c)pyran-7-carboxylic acid; MFCD00006912; Citrinin, 98%; Spectrum_000451; CITRININ [HSDB]; CITRININ [IARC]; CITRININ [MI]; Spectrum2_000734; Spectrum3_000240; Spectrum4_001804; Spectrum5_000507; BSPBio_001919; KBioGR_002411; KBioSS_000931; SPECTRUM210186; 5-18-09-00061 (Beilstein Handbook Reference); DivK1c_000646; SCHEMBL157775; SPBio_000688; DTXSID8020333; HMS502A08; KBio1_000646; KBio2_000931; KBio2_003499; KBio2_006067; KBio3_001419; NINDS_000646; HMS1923C05; CCG-39048; ZINC19795941; AKOS030254668; SDCCGMLS-0066537.P001; IDI1_000646; NCGC00160164-01; NCGC00160164-02; NCGC00160164-03; NCI60_001544; E80637; Q420354; SR-05000002496; WLN: T66 CO HV AUT&J D1 E1 G1 IVQ JQ; SR-05000002496-1; Citrinin, from Penicillium citrinum, >=98% (HPLC); Q63392265; (3R,4S)-3,4,5-Trimethyl-6-hydroxy-8-oxo-4,8-dihydro-3H-2-benzopyran-7-carboxylic acid; (3R,4S)-6-hydroxy-3,4,5-trimethyl-8-oxo-4,8-dihydro-3H-2-benzopyran-7-carboxylic acid; 3H-2-Benzopyran-7-carboxylic acid, 4,6-dihydro-8-hydroxy-3,4,5-trimethyl-6-oxo-; 8-Hydroxy-3,4,5-trimethyl-6-oxo-4,6-dihydro-3H-isochromene-7-carboxylic acid #; 3H-2-Benzopyran-7-carboxylic acid, 4,6-dihydro-8-hydroxy-3,4,5-trimethyl-6-oxo-,(3R,4S)-; 3H-2-Benzopyran-7-carboxylic acid,6-dihydro-8-hydroxy-3,4,5-trimethyl-6-oxo-, (3R-trans)-; 4,6-Dihydro-8-hydroxy-3,4,5-trimethyl-6-oxo, (3R-trans)-3H-2-benzopyran-7-carboxylic acid
|
|
| CAS | 518-75-2 | |
| PubChem CID | 54680783 | |
| ChEMBL ID | CHEMBL510139 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 250.25 | ALogp: | 1.7 |
| HBD: | 2 | HBA: | 5 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 83.8 | Aromatic Rings: | 2 |
| Heavy Atoms: | 18 | QED Weighted: | 0.697 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.628 | MDCK Permeability: | 0.00000230 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.018 |
| Human Intestinal Absorption (HIA): | 0.018 | 20% Bioavailability (F20%): | 0.004 |
| 30% Bioavailability (F30%): | 0.003 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.037 | Plasma Protein Binding (PPB): | 93.77% |
| Volume Distribution (VD): | 0.949 | Fu: | 6.20% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.227 | CYP1A2-substrate: | 0.571 |
| CYP2C19-inhibitor: | 0.033 | CYP2C19-substrate: | 0.061 |
| CYP2C9-inhibitor: | 0.065 | CYP2C9-substrate: | 0.167 |
| CYP2D6-inhibitor: | 0.03 | CYP2D6-substrate: | 0.125 |
| CYP3A4-inhibitor: | 0.051 | CYP3A4-substrate: | 0.067 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 1.593 | Half-life (T1/2): | 0.888 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.007 | Human Hepatotoxicity (H-HT): | 0.598 |
| Drug-inuced Liver Injury (DILI): | 0.984 | AMES Toxicity: | 0.402 |
| Rat Oral Acute Toxicity: | 0.217 | Maximum Recommended Daily Dose: | 0.025 |
| Skin Sensitization: | 0.367 | Carcinogencity: | 0.953 |
| Eye Corrosion: | 0.004 | Eye Irritation: | 0.336 |
| Respiratory Toxicity: | 0.872 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC002338 | 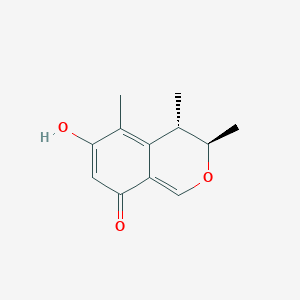 |
0.554 | D0S0LZ |  |
0.236 | ||
| ENC000945 | 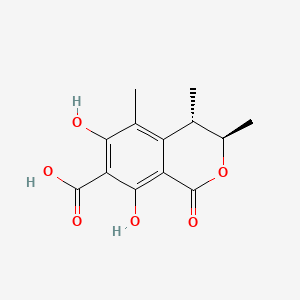 |
0.391 | D0K7LU | 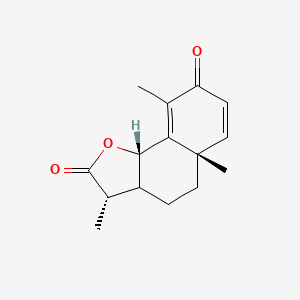 |
0.231 | ||
| ENC003584 | 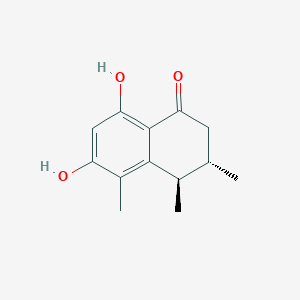 |
0.309 | D07JGT |  |
0.224 | ||
| ENC004363 | 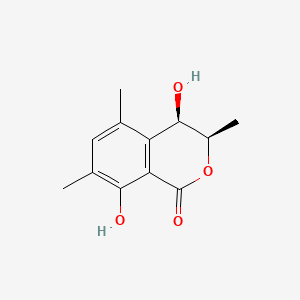 |
0.309 | D0JL2K | 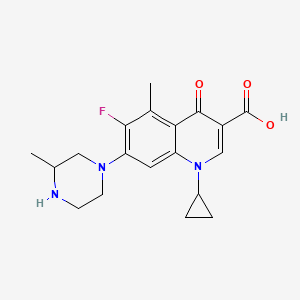 |
0.216 | ||
| ENC001362 | 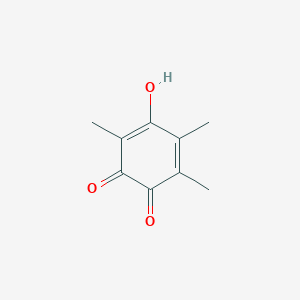 |
0.305 | D08NQZ |  |
0.213 | ||
| ENC004391 |  |
0.305 | D0YH0N |  |
0.212 | ||
| ENC000670 |  |
0.290 | D0B9EJ | 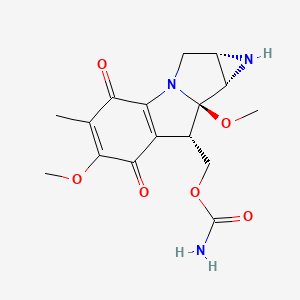 |
0.211 | ||
| ENC003525 | 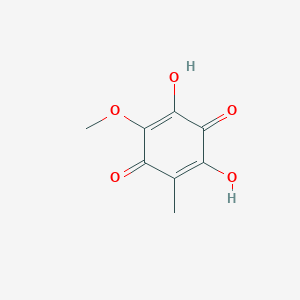 |
0.290 | D0X9ZC | 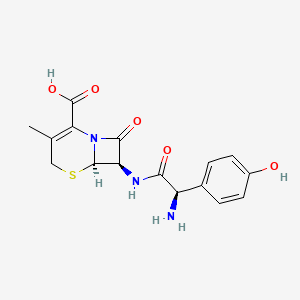 |
0.211 | ||
| ENC004991 | 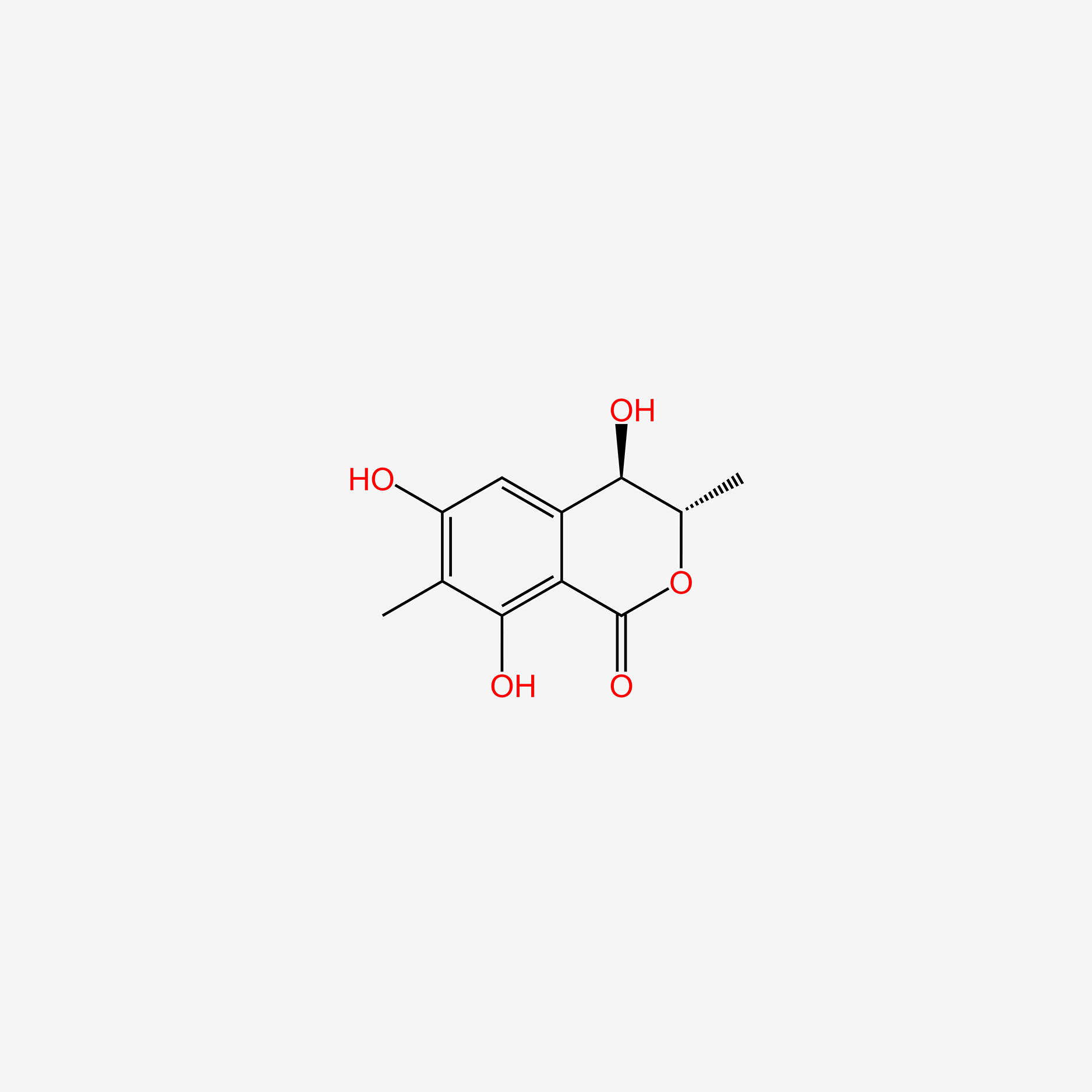 |
0.290 | D0R6RC | 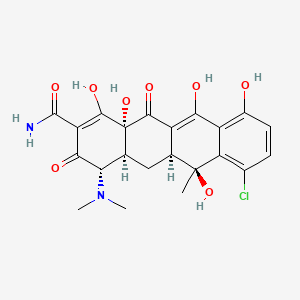 |
0.209 | ||
| ENC004969 | 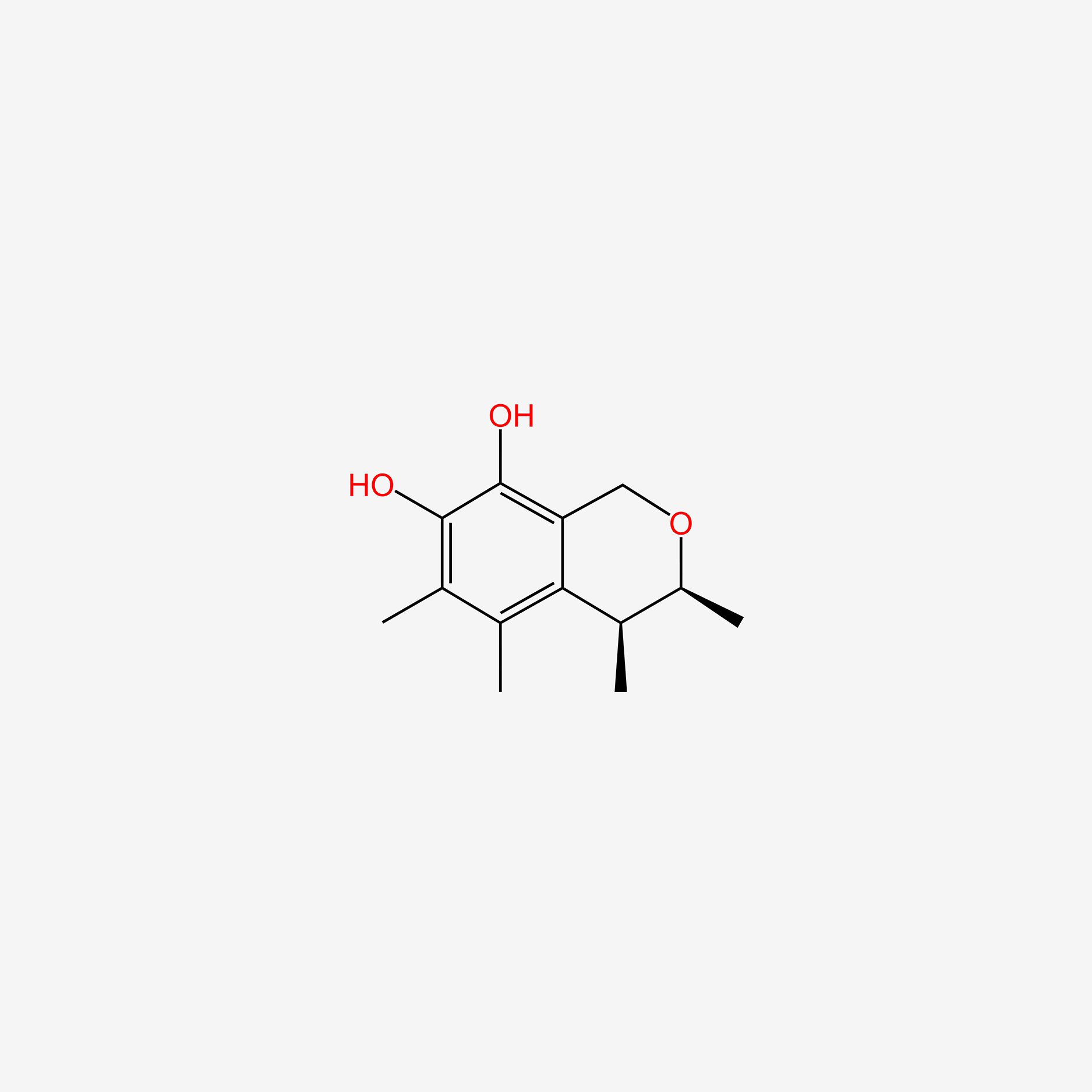 |
0.290 | D0J2NK | 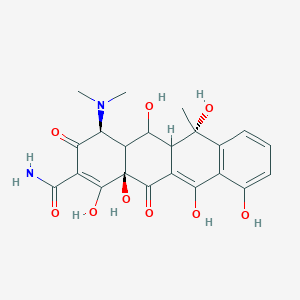 |
0.209 | ||