NPs Basic Information

|
Name |
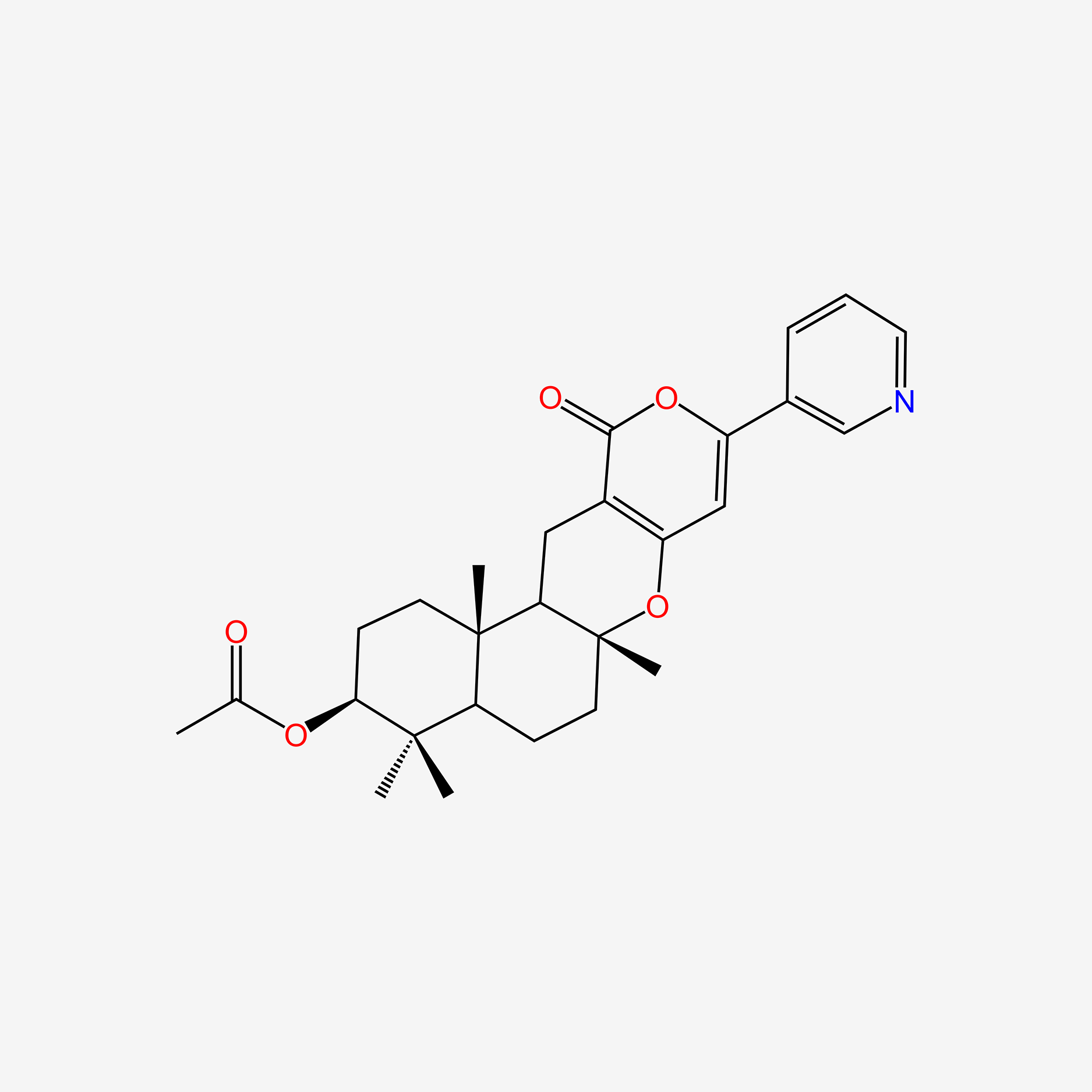
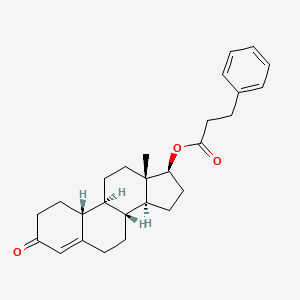
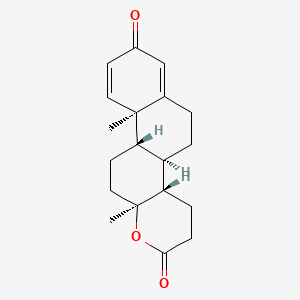
Pyripyropene F
|
| Molecular Formula | C28H35NO5 | |
| IUPAC Name* |
[(1R,2S,5S,7R,10R)-2,6,6,10-tetramethyl-16-oxo-14-pyridin-3-yl-11,15-dioxatetracyclo[8.8.0.02,7.012,17]octadeca-12(17),13-dien-5-yl] propanoate
|
|
| SMILES |
CCC(=O)O[C@H]1CC[C@]2([C@H](C1(C)C)CC[C@@]3([C@@H]2CC4=C(O3)C=C(OC4=O)C5=CN=CC=C5)C)C
|
|
| InChI |
InChI=1S/C28H35NO5/c1-6-24(30)33-23-10-11-27(4)21(26(23,2)3)9-12-28(5)22(27)14-18-20(34-28)15-19(32-25(18)31)17-8-7-13-29-16-17/h7-8,13,15-16,21-23H,6,9-12,14H2,1-5H3/t21-,22+,23-,27-,28+/m0/s1
|
|
| InChIKey |
JIHRIBKMEVTBGP-ZRLOAUAMSA-N
|
|
| Synonyms |
Pyripyropene F; GERI-BP-001B; 165467-56-1
|
|
| CAS | NA | |
| PubChem CID | 11754246 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Physi-Chem Properties
| Molecular Weight: | 465.6 | ALogp: | 5.1 |
| HBD: | 0 | HBA: | 6 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 74.7 | Aromatic Rings: | 5 |
| Heavy Atoms: | 34 | QED Weighted: | 0.537 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.885 | MDCK Permeability: | 0.00002040 |
| Pgp-inhibitor: | 0.998 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.002 | 20% Bioavailability (F20%): | 0.019 |
| 30% Bioavailability (F30%): | 0.971 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.048 | Plasma Protein Binding (PPB): | 94.43% |
| Volume Distribution (VD): | 1.634 | Fu: | 6.00% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.32 | CYP1A2-substrate: | 0.283 |
| CYP2C19-inhibitor: | 0.524 | CYP2C19-substrate: | 0.632 |
| CYP2C9-inhibitor: | 0.828 | CYP2C9-substrate: | 0.443 |
| CYP2D6-inhibitor: | 0.039 | CYP2D6-substrate: | 0.657 |
| CYP3A4-inhibitor: | 0.891 | CYP3A4-substrate: | 0.545 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 5.318 | Half-life (T1/2): | 0.183 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.472 | Human Hepatotoxicity (H-HT): | 0.641 |
| Drug-inuced Liver Injury (DILI): | 0.637 | AMES Toxicity: | 0.003 |
| Rat Oral Acute Toxicity: | 0.099 | Maximum Recommended Daily Dose: | 0.89 |
| Skin Sensitization: | 0.392 | Carcinogencity: | 0.026 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.009 |
| Respiratory Toxicity: | 0.962 |