NPs Basic Information
|
Name |
Ginsenoside Rg3
|
| Molecular Formula | C42H72O13 | |
| IUPAC Name* |
(2S,3R,4S,5S,6R)-2-[(2R,3R,4S,5S,6R)-4,5-dihydroxy-2-[[(3S,5R,8R,9R,10R,12R,13R,14R,17S)-12-hydroxy-17-[(2S)-2-hydroxy-6-methylhept-5-en-2-yl]-4,4,8,10,14-pentamethyl-2,3,5,6,7,9,11,12,13,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl]oxy]-6-(hydroxymethyl)oxan-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol
|
|
| SMILES |
CC(=CCC[C@@](C)([C@H]1CC[C@@]2([C@@H]1[C@@H](C[C@H]3[C@]2(CC[C@@H]4[C@@]3(CC[C@@H](C4(C)C)O[C@H]5[C@@H]([C@H]([C@@H]([C@H](O5)CO)O)O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O)C)C)O)C)O)C
|
|
| InChI |
InChI=1S/C42H72O13/c1-21(2)10-9-14-42(8,51)22-11-16-41(7)29(22)23(45)18-27-39(5)15-13-28(38(3,4)26(39)12-17-40(27,41)6)54-37-35(33(49)31(47)25(20-44)53-37)55-36-34(50)32(48)30(46)24(19-43)52-36/h10,22-37,43-51H,9,11-20H2,1-8H3/t22-,23+,24+,25+,26-,27+,28-,29-,30+,31+,32-,33-,34+,35+,36-,37-,39-,40+,41+,42-/m0/s1
|
|
| InChIKey |
RWXIFXNRCLMQCD-JBVRGBGGSA-N
|
|
| Synonyms |
Ginsenoside Rg3; 14197-60-5; 20s-ginsenoside rg3; (20S)-Propanaxadiol; 20S-propanaxadiol; (20S)-ginsenoside Rg3; S-Ginsenoside Rg3; 20(S)-Ginsenoside Rg3; 20(S)-Ginsenoside-Rg3; 20(S)-Propanaxidiol; CHEMBL398412; CHEBI:67991; (2S,3R,4S,5S,6R)-2-[(2R,3R,4S,5S,6R)-4,5-dihydroxy-2-[[(3S,5R,8R,9R,10R,12R,13R,14R,17S)-12-hydroxy-17-[(2S)-2-hydroxy-6-methylhept-5-en-2-yl]-4,4,8,10,14-pentamethyl-2,3,5,6,7,9,11,12,13,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl]oxy]-6-(hydroxymethyl)oxan-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol; 11019-45-7; 227D367Y57; (R)Ginsenoside-Rg3; ginsenoside 20-rg3; ginsenoside rg3, (s)-; ginsenoside rg3, (+)-; UNII-227D367Y57; Rg3; MFCD06410950; Ginsenoside Rg3,(S); 2'(R)-Ginsenoside-Rg3; GTPL7658; GINSENOSIDE RG3 [WHO-DD]; DTXSID101316982; HMS3886B14; BDBM50317537; Ginsenoside Rg3, analytical standard; s9022; ZINC96085900; Ginsenoside Rg3, >=98% (HPLC); AKOS037514674; CCG-270472; (3beta,12beta)-12,20-dihydroxydammar-24-en-3-yl 2-O-beta-D-glucopyranosyl-beta-D-glucopyranoside; AS-56617; Dammar-24-ene-12-beta,20-diol, 3-beta-((2-O-beta-D-glucopyranosyl-beta-D-glucopyransoyl)oxy)-; C20778; 197G605; Q-100154; Q27077807; .BETA.-D-GLUCOPYRANOSIDE, (3.BETA.,12.BETA.)-12,20-DIHYDROXYDAMMAR-24-EN-3-YL 2-O-.BETA.-D-GLUCOPYRANOSYL-; 3-O-.BETA.-D-GLUCOPYRANOSYL-(1->2)-.BETA.-D-GLUCOPYRANOSYLDAMMAR-24-ENE-3.BETA.,12.BETA.,20S-TRIOL; beta-D-Glucopyranoside, (3-beta,12-beta)-12,20-dihydroxydammar-24-en-3-yl 2-O-beta-D-glucopyranosyl-; dammar-24-ene-12beta,20-diol, 3-beta-((2-O-beta-D-glucopyranosyl-beta-D-glucopyransoyl)oxy)-
|
|
| CAS | 14197-60-5 | |
| PubChem CID | 9918693 | |
| ChEMBL ID | CHEMBL398412 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 785.0 | ALogp: | 4.0 |
| HBD: | 9 | HBA: | 13 |
| Rotatable Bonds: | 10 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 219.0 | Aromatic Rings: | 6 |
| Heavy Atoms: | 55 | QED Weighted: | 0.115 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.461 | MDCK Permeability: | 0.00004740 |
| Pgp-inhibitor: | 0.998 | Pgp-substrate: | 0.009 |
| Human Intestinal Absorption (HIA): | 0.839 | 20% Bioavailability (F20%): | 0.9 |
| 30% Bioavailability (F30%): | 0.986 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.008 | Plasma Protein Binding (PPB): | 81.38% |
| Volume Distribution (VD): | 0.357 | Fu: | 7.48% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.001 | CYP1A2-substrate: | 0.1 |
| CYP2C19-inhibitor: | 0.001 | CYP2C19-substrate: | 0.48 |
| CYP2C9-inhibitor: | 0.004 | CYP2C9-substrate: | 0.024 |
| CYP2D6-inhibitor: | 0.002 | CYP2D6-substrate: | 0.063 |
| CYP3A4-inhibitor: | 0.04 | CYP3A4-substrate: | 0.065 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 0.783 | Half-life (T1/2): | 0.659 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.098 | Human Hepatotoxicity (H-HT): | 0.142 |
| Drug-inuced Liver Injury (DILI): | 0.007 | AMES Toxicity: | 0.051 |
| Rat Oral Acute Toxicity: | 0.118 | Maximum Recommended Daily Dose: | 0.02 |
| Skin Sensitization: | 0.623 | Carcinogencity: | 0.009 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.015 |
| Respiratory Toxicity: | 0.743 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
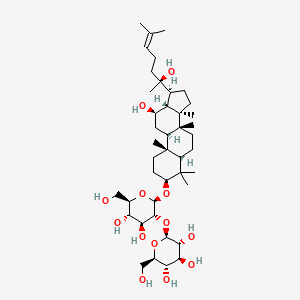
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001938 | 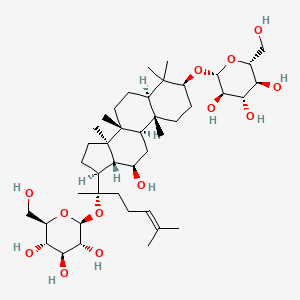 |
0.862 | D07QQD | 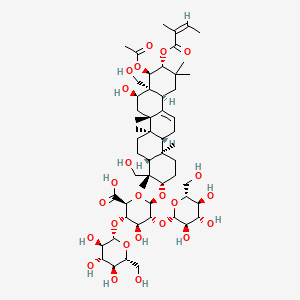 |
0.463 | ||
| ENC000865 | 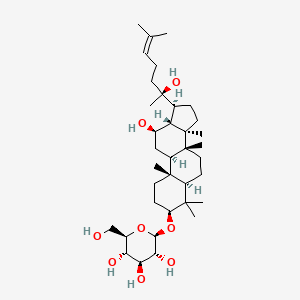 |
0.797 | D04MRG |  |
0.461 | ||
| ENC002180 |  |
0.781 | D0P2IT |  |
0.424 | ||
| ENC002655 |  |
0.725 | D04RYU |  |
0.402 | ||
| ENC001894 |  |
0.688 | D03MTN |  |
0.393 | ||
| ENC002245 |  |
0.688 | D07ORO |  |
0.372 | ||
| ENC001933 |  |
0.671 | D0YV1Q |  |
0.349 | ||
| ENC002246 |  |
0.665 | D0A8RX |  |
0.345 | ||
| ENC001918 |  |
0.605 | D0Y3MO |  |
0.342 | ||
| ENC002152 |  |
0.488 | D0P6IK |  |
0.340 | ||