NPs Basic Information

|
Name |
(11S,14S)-Cyclo-(L-Trp-L-Phe)
|
| Molecular Formula | C20H19N3O2 | |
| IUPAC Name* |
(3S,6S)-3-benzyl-6-(1H-indol-3-ylmethyl)piperazine-2,5-dione
|
|
| SMILES |
C1=CC=C(C=C1)C[C@H]2C(=O)N[C@H](C(=O)N2)CC3=CNC4=CC=CC=C43
|
|
| InChI |
InChI=1S/C20H19N3O2/c24-19-17(10-13-6-2-1-3-7-13)22-20(25)18(23-19)11-14-12-21-16-9-5-4-8-15(14)16/h1-9,12,17-18,21H,10-11H2,(H,22,25)(H,23,24)/t17-,18-/m0/s1
|
|
| InChIKey |
CUVKAUWOMPJEMI-ROUUACIJSA-N
|
|
| Synonyms |
(11S,14S)-Cyclo-(L-Trp-L-Phe); Cyclo(-Phe-Trp); CHEBI:68230; (3S,6S)-3-benzyl-6-(1H-indol-3-ylmethyl)piperazine-2,5-dione; Isorugulosuvine; Cyclo(-L-Phe-L-Trp); 6521-48-8; Cyclo(Phe-Trp-); Cyclo(L-Trp-L-Phe); MLS002704309; CHEMBL190059; DTXSID901017851; ZINC4899716; SMR001571011; C22142; Q27136723
|
|
| CAS | 6521-48-8 | |
| PubChem CID | 7408486 | |
| ChEMBL ID | CHEMBL190059 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 333.4 | ALogp: | 2.8 |
| HBD: | 3 | HBA: | 2 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 74.0 | Aromatic Rings: | 4 |
| Heavy Atoms: | 25 | QED Weighted: | 0.686 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.69 | MDCK Permeability: | 0.00002730 |
| Pgp-inhibitor: | 0.017 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.867 | 20% Bioavailability (F20%): | 0.957 |
| 30% Bioavailability (F30%): | 0.989 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.226 | Plasma Protein Binding (PPB): | 94.20% |
| Volume Distribution (VD): | 0.623 | Fu: | 8.08% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.33 | CYP1A2-substrate: | 0.124 |
| CYP2C19-inhibitor: | 0.897 | CYP2C19-substrate: | 0.066 |
| CYP2C9-inhibitor: | 0.678 | CYP2C9-substrate: | 0.842 |
| CYP2D6-inhibitor: | 0.477 | CYP2D6-substrate: | 0.796 |
| CYP3A4-inhibitor: | 0.922 | CYP3A4-substrate: | 0.208 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 4.001 | Half-life (T1/2): | 0.741 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.268 | Human Hepatotoxicity (H-HT): | 0.394 |
| Drug-inuced Liver Injury (DILI): | 0.415 | AMES Toxicity: | 0.697 |
| Rat Oral Acute Toxicity: | 0.779 | Maximum Recommended Daily Dose: | 0.833 |
| Skin Sensitization: | 0.204 | Carcinogencity: | 0.118 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.011 |
| Respiratory Toxicity: | 0.121 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC004531 |  |
1.000 | D02DMQ | 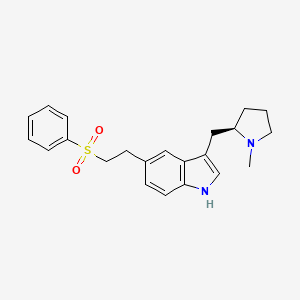 |
0.373 | ||
| ENC001911 |  |
0.762 | D08FTG | 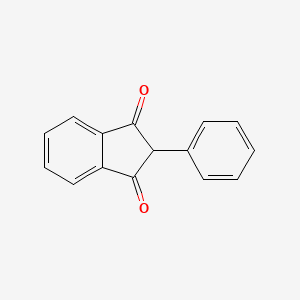 |
0.371 | ||
| ENC004971 |  |
0.747 | D02XIY | 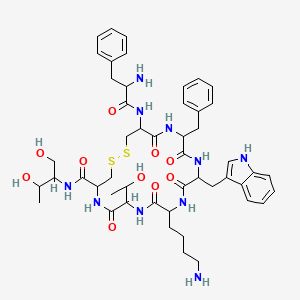 |
0.367 | ||
| ENC002149 |  |
0.616 | D0E4DW | 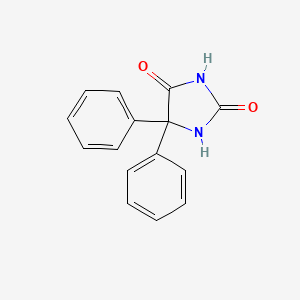 |
0.362 | ||
| ENC004711 | 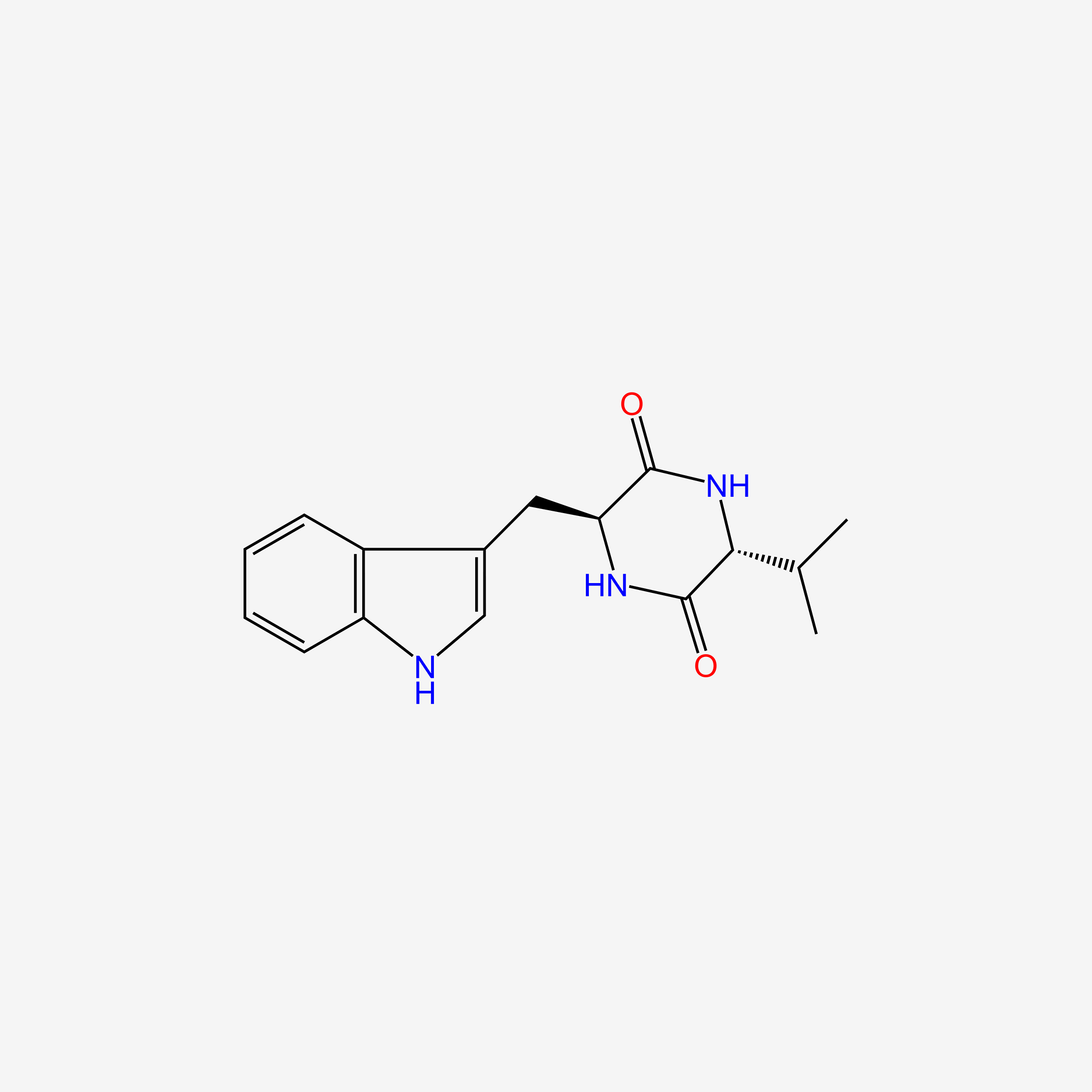 |
0.571 | D0BV3J | 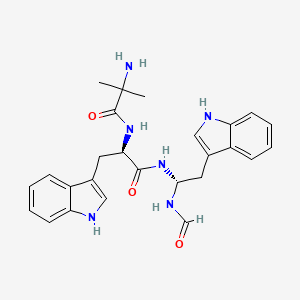 |
0.354 | ||
| ENC005471 |  |
0.550 | D0X9PF | 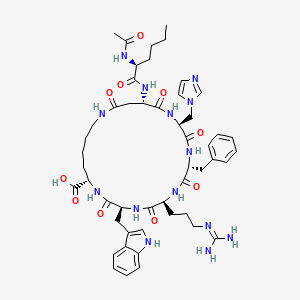 |
0.351 | ||
| ENC005997 |  |
0.536 | D0G1VX |  |
0.348 | ||
| ENC001905 | 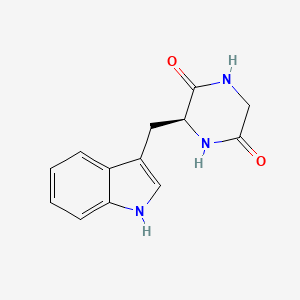 |
0.524 | D0B1FE | 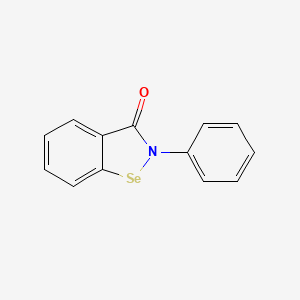 |
0.348 | ||
| ENC001909 | 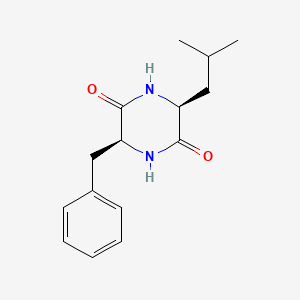 |
0.518 | D0QV5T | 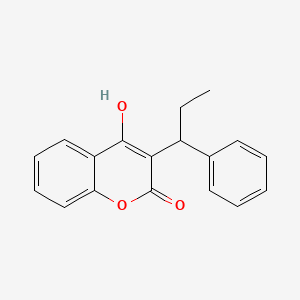 |
0.343 | ||
| ENC003208 |  |
0.517 | D0E3OF | 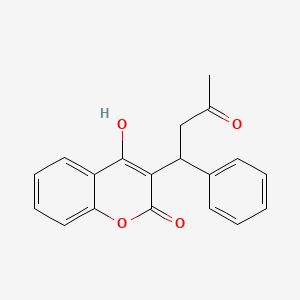 |
0.340 | ||