NPs Basic Information
|
Name |
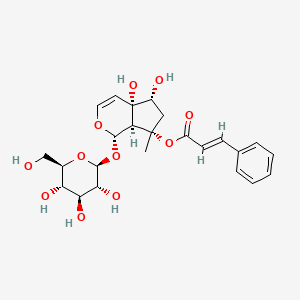
Harpagoside
|
| Molecular Formula | C24H30O11 | |
| IUPAC Name* |
[(1S,4aS,5R,7S,7aS)-4a,5-dihydroxy-7-methyl-1-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-1,5,6,7a-tetrahydrocyclopenta[c]pyran-7-yl] (E)-3-phenylprop-2-enoate
|
|
| SMILES |
C[C@@]1(C[C@H]([C@]2([C@@H]1[C@@H](OC=C2)O[C@H]3[C@@H]([C@H]([C@@H]([C@H](O3)CO)O)O)O)O)O)OC(=O)/C=C/C4=CC=CC=C4
|
|
| InChI |
InChI=1S/C24H30O11/c1-23(35-16(27)8-7-13-5-3-2-4-6-13)11-15(26)24(31)9-10-32-22(20(23)24)34-21-19(30)18(29)17(28)14(12-25)33-21/h2-10,14-15,17-22,25-26,28-31H,11-12H2,1H3/b8-7+/t14-,15-,17-,18+,19-,20-,21+,22+,23+,24-/m1/s1
|
|
| InChIKey |
KVRQGMOSZKPBNS-FMHLWDFHSA-N
|
|
| Synonyms |
Harpagoside; 19210-12-9; E-harpagoside; 8KGS1DC5ZU; CHEBI:5625; [(1S,4aS,5R,7S,7aS)-4a,5-dihydroxy-7-methyl-1-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-1,5,6,7a-tetrahydrocyclopenta[c]pyran-7-yl] (E)-3-phenylprop-2-enoate; SMR001233395; UNII-8KGS1DC5ZU; Harpaside; EINECS 242-881-6; Prestwick3_000988; HARPAGOSIDE [INCI]; BSPBio_001055; HARPAGOSIDE [WHO-DD]; MLS002154086; MLS002473324; SCHEMBL893387; BPBio1_001161; CHEMBL516702; MEGxp0_000469; Harpagoside, analytical standard; ACon0_000056; ACon1_000134; DTXSID101032528; HMS2098E17; HMS2231N12; HY-N0396; ZINC8214398; MFCD00017415; s9171; AKOS015896715; CCG-269650; LMPR0102070010; NCGC00179325-01; NCGC00179325-02; NCGC00179325-04; AC-34268; AS-56071; AB00513986; CS-0008931; C09783; H10494; 210H129; A880338; Q-100231; BRD-K07996107-001-01-7; BRD-K07996107-001-03-3; Harpagoside, primary pharmaceutical reference standard; Q25099323; Harpagoside, European Pharmacopoeia (EP) Reference Standard; (1S,4aS,5R,7S,7aS)-4a,5-Dihydroxy-7-methyl-1-(((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)-1,4a,5,6,7,7a-hexahydrocyclopenta[c]pyran-7-yl cinnamate; (1S,4aS,5R,7S,7aS)-4a,5-dihydroxy-7-methyl-1-((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yloxy)-1,4a,5,6,7,7a-hexahydrocyclopenta[c]pyran-7-yl cinnamate; (1S-(1alpha,4aalpha,5alpha,7alpha(E),7aalpha))-1,4a,5,6,7,7a-Hexahydro-4a,5-dihydroxy-7-methyl-7-((allyl-1-oxo-3-phenyl)oxy)cyclopenta(c)pyran-1-yl-beta-D-glucopyranoside; .BETA.-D-GLUCOPYRANOSIDE, (1S,4AS,5R,7S,7AS)-1,4A,5,6,7,7A-HEXAHYDRO-4A,5-DIHYDROXY-7-METHYL-7-(((2E)-1-OXO-3-PHENYL-2-PROPENYL)OXY)CYCLOPENTA(C)PYRAN-1-YL; NCGC00179325-04_C24H30O11_(1S,4aS,5R,7S,7aS)-1-(beta-D-Glucopyranosyloxy)-4a,5-dihydroxy-7-methyl-1,4a,5,6,7,7a-hexahydrocyclopenta[c]pyran-7-yl (2E)-3-phenylacrylate
|
|
| CAS | 19210-12-9 | |
| PubChem CID | 5281542 | |
| ChEMBL ID | CHEMBL516702 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 494.5 | ALogp: | -0.6 |
| HBD: | 6 | HBA: | 11 |
| Rotatable Bonds: | 7 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 175.0 | Aromatic Rings: | 4 |
| Heavy Atoms: | 35 | QED Weighted: | 0.218 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.794 | MDCK Permeability: | 0.00005790 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.89 |
| Human Intestinal Absorption (HIA): | 0.377 | 20% Bioavailability (F20%): | 0.059 |
| 30% Bioavailability (F30%): | 0.997 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.119 | Plasma Protein Binding (PPB): | 58.24% |
| Volume Distribution (VD): | 0.504 | Fu: | 26.32% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.023 | CYP1A2-substrate: | 0.07 |
| CYP2C19-inhibitor: | 0.016 | CYP2C19-substrate: | 0.178 |
| CYP2C9-inhibitor: | 0.006 | CYP2C9-substrate: | 0.1 |
| CYP2D6-inhibitor: | 0.011 | CYP2D6-substrate: | 0.094 |
| CYP3A4-inhibitor: | 0.241 | CYP3A4-substrate: | 0.066 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 1.229 | Half-life (T1/2): | 0.626 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.03 | Human Hepatotoxicity (H-HT): | 0.203 |
| Drug-inuced Liver Injury (DILI): | 0.1 | AMES Toxicity: | 0.151 |
| Rat Oral Acute Toxicity: | 0.226 | Maximum Recommended Daily Dose: | 0.088 |
| Skin Sensitization: | 0.53 | Carcinogencity: | 0.906 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.038 |
| Respiratory Toxicity: | 0.981 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC005608 | 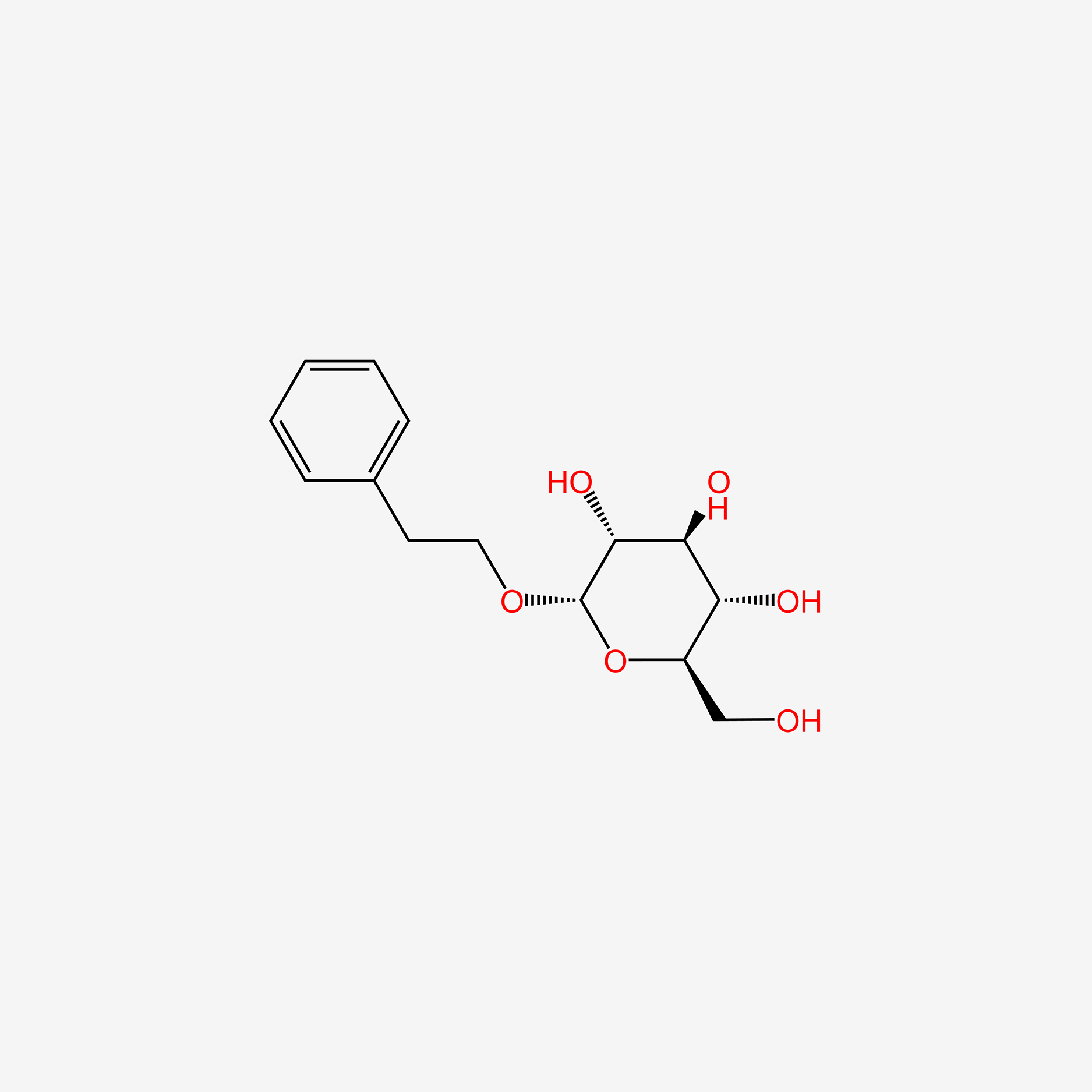 |
0.391 | D06BQU | 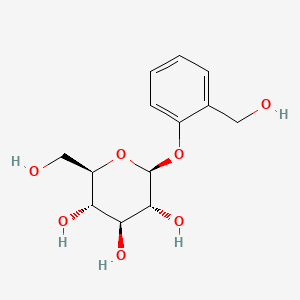 |
0.369 | ||
| ENC003351 | 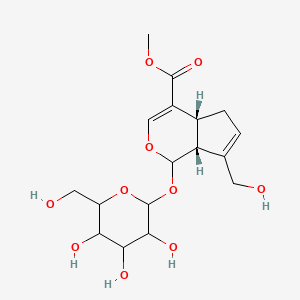 |
0.390 | D0T5BC |  |
0.336 | ||
| ENC002269 |  |
0.358 | D02HYK |  |
0.297 | ||
| ENC004291 |  |
0.354 | D0YV1Q | 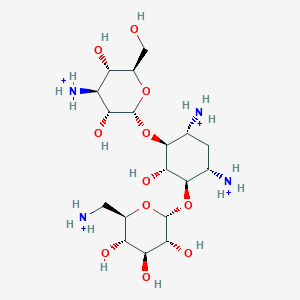 |
0.285 | ||
| ENC005528 | 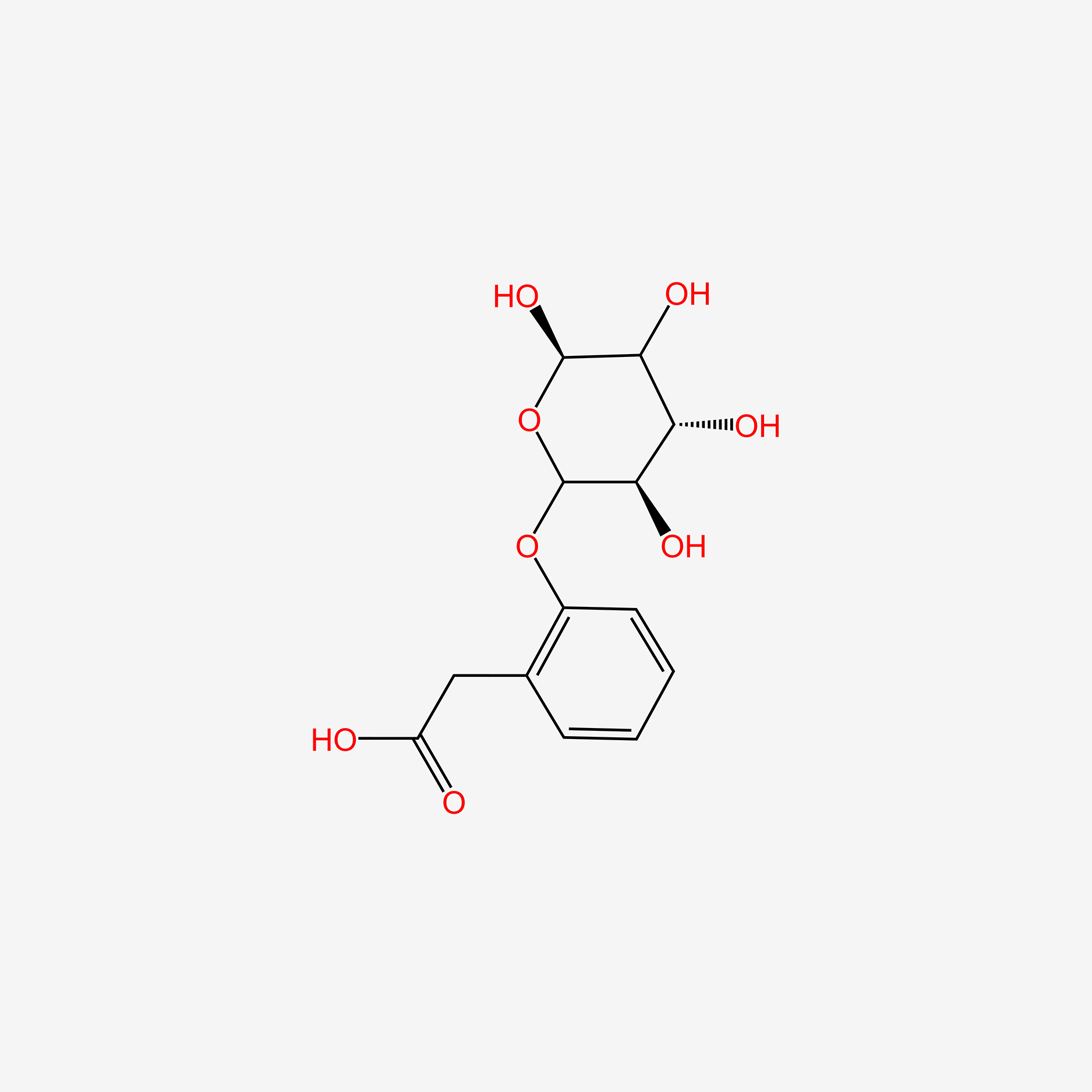 |
0.328 | D0PI3Z | 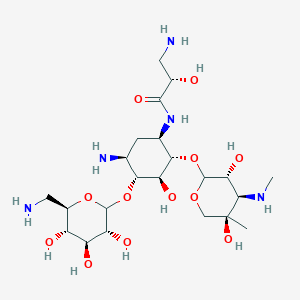 |
0.280 | ||
| ENC002582 | 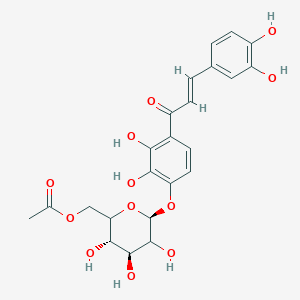 |
0.315 | D0Y3MO |  |
0.275 | ||
| ENC002949 |  |
0.310 | D04RYU |  |
0.270 | ||
| ENC004475 |  |
0.307 | D0N0EQ |  |
0.269 | ||
| ENC004476 |  |
0.302 | D01TNW |  |
0.268 | ||
| ENC003397 |  |
0.301 | D0H3KI |  |
0.267 | ||