NPs Basic Information
|
Name |
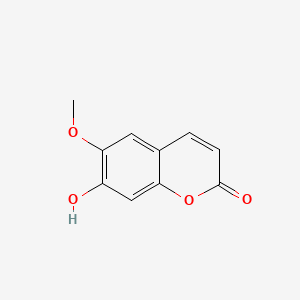
Scopoletin
|
| Molecular Formula | C10H8O4 | |
| IUPAC Name* |
7-hydroxy-6-methoxychromen-2-one
|
|
| SMILES |
COC1=C(C=C2C(=C1)C=CC(=O)O2)O
|
|
| InChI |
InChI=1S/C10H8O4/c1-13-9-4-6-2-3-10(12)14-8(6)5-7(9)11/h2-5,11H,1H3
|
|
| InChIKey |
RODXRVNMMDRFIK-UHFFFAOYSA-N
|
|
| Synonyms |
scopoletin; 92-61-5; Gelseminic acid; 7-Hydroxy-6-methoxy-2H-chromen-2-one; 6-Methylesculetin; Chrysatropic acid; Scopoletine; 7-Hydroxy-6-methoxycoumarin; Murrayetin; Scopoletol; Escopoletin; 6-O-Methylesculetin; 6-Methoxy-7-hydroxycoumarin; 7-Hydroxy-6-methoxy-2H-1-benzopyran-2-one; 2H-1-Benzopyran-2-one, 7-hydroxy-6-methoxy-; 6-Methoxyumbelliferone; Esculetin 6-methyl ether; 7-hydroxy-6-methoxychromen-2-one; beta-Methylesculetin; Buxuletin; .beta.-Methylesculetin; Esculetin-6-methyl ether; Baogongteng B; COUMARIN, 7-HYDROXY-6-METHOXY-; NSC 405647; KLF1HS0SXJ; 7-hydroxy-6-methoxy-chromen-2-one; NSC405647; NSC-405647; CHEMBL71851; CHEBI:17488; Gelseminic acid;Chrysatropic acid; TNP00096; Chrysotropic Acid; Acid, Gelseminic; Acid, Chrysotropic; SMR000112541; CCRIS 3592; SR-01000841273; UNII-KLF1HS0SXJ; EINECS 202-171-9; MFCD00006872; BRN 0156296; b-Methylaesculetin; beta -methylesculetin; COPOLETIN; Scopoletin, >=99%; SCOPOLETIN [MI]; Prestwick0_000962; Prestwick1_000962; Prestwick2_000962; Prestwick3_000962; Spectrum2_001207; Spectrum3_001532; Spectrum4_001054; Spectrum5_000654; Aesculetin 6-methyl ether; SCOPOLETIN [USP-RS]; BIDD:PXR0125; BSPBio_000963; BSPBio_002944; KBioGR_001348; 5-18-03-00203 (Beilstein Handbook Reference); 7-hydroxy-6-methoxy-coumarin; MLS002154074; MLS002472878; DivK1c_000720; SCHEMBL147702; SPECTRUM1502242; 7-hydroxy 6-methoxy coumarine; SPBio_000994; SPBio_002884; Scopoletin, analytical standard; BPBio1_001061; MEGxp0_001192; DTXSID0075368; ACon1_000143; HMS502D22; KBio1_000720; KBio3_002444; ZINC57733; NINDS_000720; HMS1571A05; HMS1921N16; HMS2098A05; HMS2268G04; HMS3885K10; ALBB-023369; BCP13342; HY-N0342; 6-methoxy-7-oxidanyl-chromen-2-one; BDBM50156693; CCG-39140; s3881; STL570289; TD8126; AKOS000277133; CS-5791; CAS-92-61-5; IDI1_000720; 7-hydroxy-6-methoxy-1-benzopyran-2-one; NCGC00016349-01; NCGC00016349-02; NCGC00016349-03; NCGC00016349-04; NCGC00016349-05; NCGC00016349-06; NCGC00016349-07; NCGC00016349-08; NCGC00094973-01; NCGC00094973-02; NCGC00094973-03; AC-34125; AS-65759; NCI60_003834; 7-Hydroxy-6-methoxy-2H-chromen-2-one #; DB-050232; AB00443525; FT-0631451; S0367; C01752; S-2000; 006S872; A844290; Q2472366; SR-01000841273-3; SR-01000841273-4; BRD-K96163925-001-06-5; BRD-K96163925-001-09-9; 2H-1-Benzopyran-2-one, 7-hydroxy-6-methoxy- (9CI); 0B4B9FAA-686D-4977-AA08-65F8E4F1977C; SCOPOLETIN (CONSTITUENT OF STINGING NETTLE) [DSC]; Scopoletin, United States Pharmacopeia (USP) Reference Standard; T83
|
|
| CAS | 92-61-5 | |
| PubChem CID | 5280460 | |
| ChEMBL ID | CHEMBL71851 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 192.17 | ALogp: | 1.5 |
| HBD: | 1 | HBA: | 4 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 55.8 | Aromatic Rings: | 2 |
| Heavy Atoms: | 14 | QED Weighted: | 0.702 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.725 | MDCK Permeability: | 0.00001630 |
| Pgp-inhibitor: | 0.003 | Pgp-substrate: | 0.945 |
| Human Intestinal Absorption (HIA): | 0.009 | 20% Bioavailability (F20%): | 0.236 |
| 30% Bioavailability (F30%): | 0.998 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.157 | Plasma Protein Binding (PPB): | 83.62% |
| Volume Distribution (VD): | 0.689 | Fu: | 19.39% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.971 | CYP1A2-substrate: | 0.948 |
| CYP2C19-inhibitor: | 0.181 | CYP2C19-substrate: | 0.078 |
| CYP2C9-inhibitor: | 0.052 | CYP2C9-substrate: | 0.85 |
| CYP2D6-inhibitor: | 0.487 | CYP2D6-substrate: | 0.878 |
| CYP3A4-inhibitor: | 0.305 | CYP3A4-substrate: | 0.292 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 13.312 | Half-life (T1/2): | 0.85 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.09 | Human Hepatotoxicity (H-HT): | 0.245 |
| Drug-inuced Liver Injury (DILI): | 0.814 | AMES Toxicity: | 0.06 |
| Rat Oral Acute Toxicity: | 0.069 | Maximum Recommended Daily Dose: | 0.372 |
| Skin Sensitization: | 0.555 | Carcinogencity: | 0.595 |
| Eye Corrosion: | 0.358 | Eye Irritation: | 0.978 |
| Respiratory Toxicity: | 0.233 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001561 | 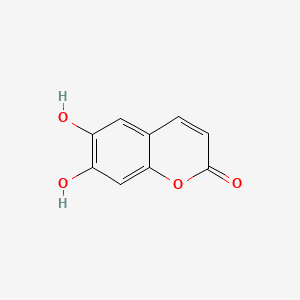 |
0.667 | D08SKH | 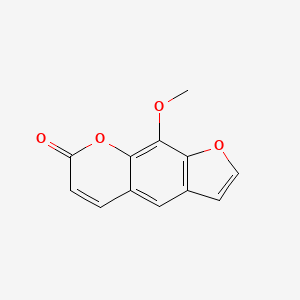 |
0.417 | ||
| ENC001524 | 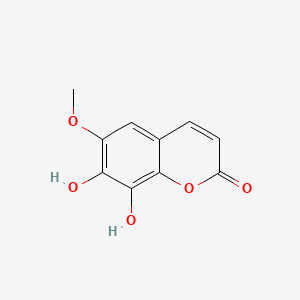 |
0.569 | D0E9CD |  |
0.408 | ||
| ENC001623 | 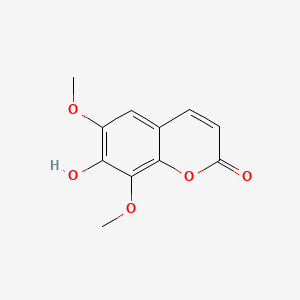 |
0.537 | D07MGA |  |
0.351 | ||
| ENC001562 | 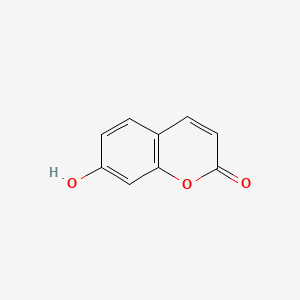 |
0.521 | D06GCK |  |
0.350 | ||
| ENC001367 | 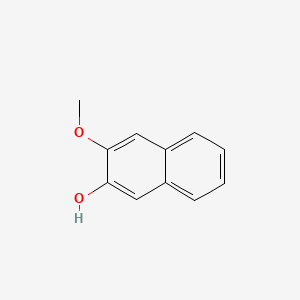 |
0.490 | D0DJ1B |  |
0.303 | ||
| ENC001539 | 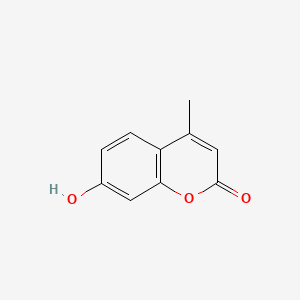 |
0.442 | D00CSQ | 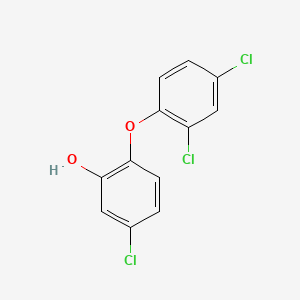 |
0.284 | ||
| ENC005717 | 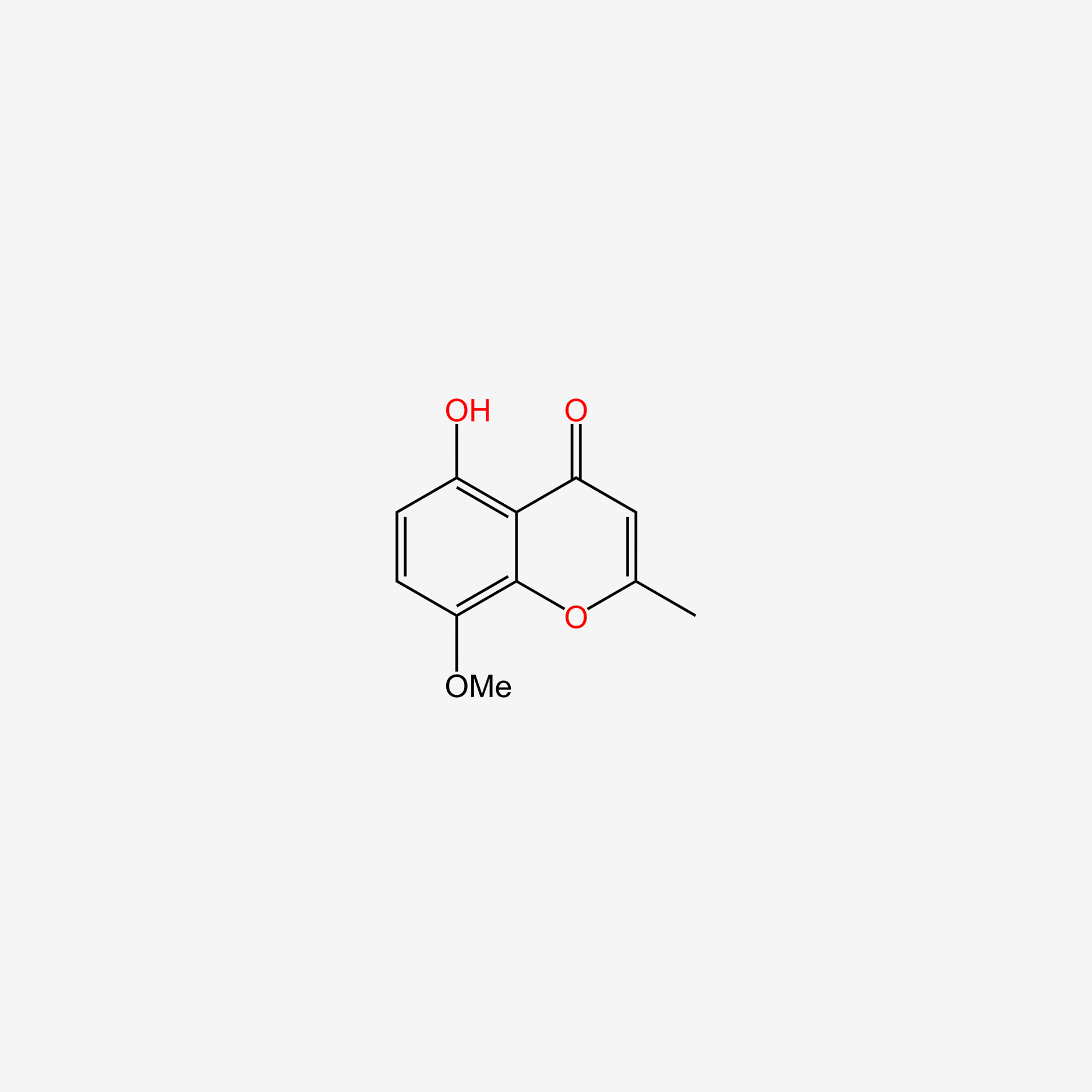 |
0.429 | D05CKR | 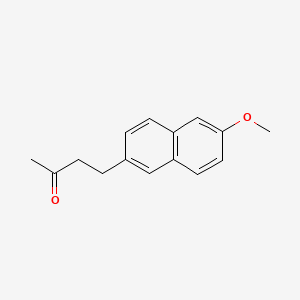 |
0.279 | ||
| ENC005716 | 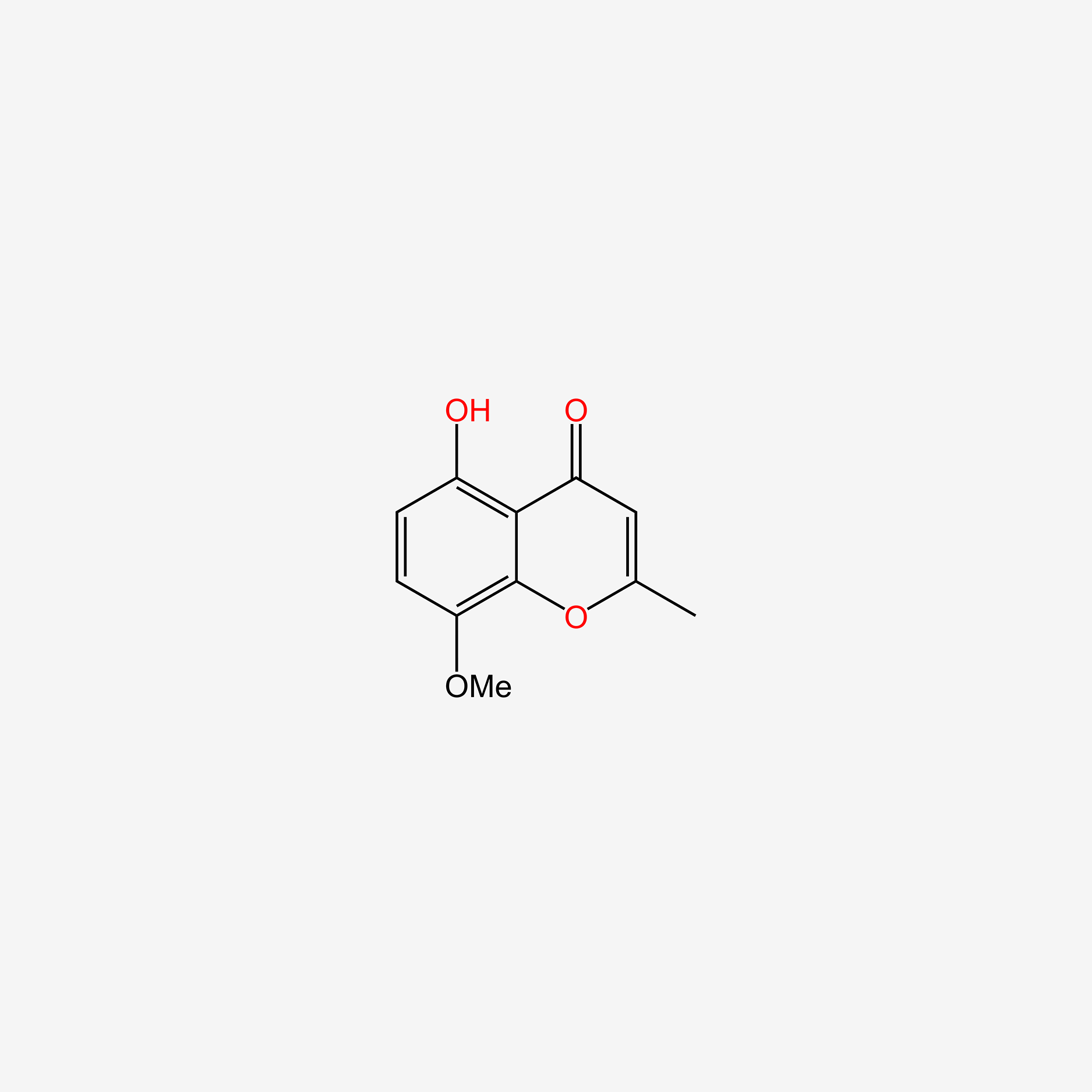 |
0.429 | D04AIT |  |
0.276 | ||
| ENC000078 | 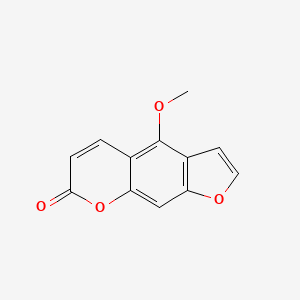 |
0.417 | D08ZEB | 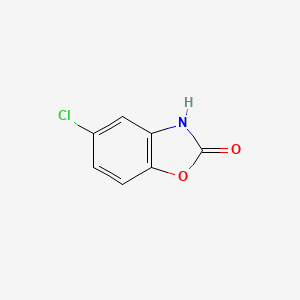 |
0.273 | ||
| ENC002901 | 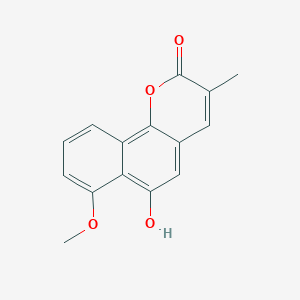 |
0.415 | D0K8KX |  |
0.269 | ||