NPs Basic Information
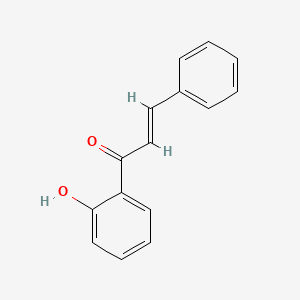
|
Name |
2'-Hydroxychalcone
|
| Molecular Formula | C15H12O2 | |
| IUPAC Name* |
(E)-1-(2-hydroxyphenyl)-3-phenylprop-2-en-1-one
|
|
| SMILES |
C1=CC=C(C=C1)/C=C/C(=O)C2=CC=CC=C2O
|
|
| InChI |
InChI=1S/C15H12O2/c16-14-9-5-4-8-13(14)15(17)11-10-12-6-2-1-3-7-12/h1-11,16H/b11-10+
|
|
| InChIKey |
AETKQQBRKSELEL-ZHACJKMWSA-N
|
|
| Synonyms |
2'-Hydroxychalcone; 1214-47-7; 888-12-0; o-Hydroxychalcone; (E)-2'-Hydroxychalcone; 1-(2-hydroxyphenyl)-3-phenylprop-2-en-1-one; Chalcone, 2'-hydroxy-; 1-(2-Hydroxyphenyl)-3-phenyl-2-propen-1-one; (E)-1-(2-hydroxyphenyl)-3-phenylprop-2-en-1-one; (2E)-1-(2-hydroxyphenyl)-3-phenylprop-2-en-1-one; CCRIS 7796; NSC 18939; Acrylophenone, 2'-hydroxy-3-phenyl-; 2-PROPEN-1-ONE, 1-(2-HYDROXYPHENYL)-3-PHENYL-; 1-(2-Hydroxyphenyl)-3-phenyl-2-propenone; VY06DZ94OC; CHEBI:27916; CMLDBU00002599; NSC-18939; (2E)-1-(2-Hydroxyphenyl)-3-phenyl-2-propen-1-one; NSC-170284; 2-propen-1-one, 1-(2-hydroxyphenyl)-3-phenyl-, (2E)-; EINECS 214-928-0; UNII-VY06DZ94OC; BRN 0976324; o-Cinnamoylphenol; Chalcone, 6; EINECS 212-962-0; 2''-Hydroxychalcone; 2\'-Hydroxychalcone; 1-(2-Hydroxy-phenyl)-3-phenyl-propenone; trans-2'-Hydroxychalcone; 2-Hydroxybenzalacetophenone; 2-08-00-00220 (Beilstein Handbook Reference); MLS000438917; CHEMBL32147; 2-Benzal-2'-hydroxyacetophenone; BDBM86007; RVC-556; DTXSID001313416; HMS2206A17; NSC18939; 2-Benzylidene-2'-hydroxyacetophenone; MFCD00016441; NSC170284; STL015897; ZINC12359992; AKOS002346618; AKOS025311108; CCG-102277; CS-W013065; CHALCONE, 2'-HYDROXY-, (E)-; NCGC00017803-02; NCGC00017803-03; NCGC00017803-06; NCGC00161072-01; NCGC00161072-02; AS-16009; LS-14376; SMR000112946; H0385; 3-Phenyl-1-(2-hydroxyphenyl)-2-propen-1-one; C09321; H-4450; 1-(2-Hydroxyphenyl)-3-phenyl-2-propenone, 98%; AE-848/32054053; SR-01000721424; SR-01000721424-2; W-109608; (E)-1-(2-hydroxyphenyl)-3-phenyl-prop-2-en-1-one; BRD-K52053379-001-02-0; Q27103405; 2-Propen-1-one, 1-(2-hydroxyphenyl)-3-phenyl-, (E)-; (5Z)-5-[(2E)-3-Phenyl-1-hydroxyallylidene]-1,3-cyclohexadiene-6-one
|
|
| CAS | 888-12-0 | |
| PubChem CID | 638276 | |
| ChEMBL ID | CHEMBL32147 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 224.25 | ALogp: | 3.9 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 3 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 37.3 | Aromatic Rings: | 2 |
| Heavy Atoms: | 17 | QED Weighted: | 0.63 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.724 | MDCK Permeability: | 0.00001350 |
| Pgp-inhibitor: | 0.004 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.016 | 20% Bioavailability (F20%): | 0.843 |
| 30% Bioavailability (F30%): | 0.23 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.368 | Plasma Protein Binding (PPB): | 99.89% |
| Volume Distribution (VD): | 0.487 | Fu: | 0.86% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.987 | CYP1A2-substrate: | 0.113 |
| CYP2C19-inhibitor: | 0.903 | CYP2C19-substrate: | 0.064 |
| CYP2C9-inhibitor: | 0.764 | CYP2C9-substrate: | 0.942 |
| CYP2D6-inhibitor: | 0.735 | CYP2D6-substrate: | 0.712 |
| CYP3A4-inhibitor: | 0.359 | CYP3A4-substrate: | 0.182 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.663 | Half-life (T1/2): | 0.589 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.046 | Human Hepatotoxicity (H-HT): | 0.022 |
| Drug-inuced Liver Injury (DILI): | 0.514 | AMES Toxicity: | 0.567 |
| Rat Oral Acute Toxicity: | 0.047 | Maximum Recommended Daily Dose: | 0.086 |
| Skin Sensitization: | 0.919 | Carcinogencity: | 0.713 |
| Eye Corrosion: | 0.611 | Eye Irritation: | 0.994 |
| Respiratory Toxicity: | 0.21 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001523 | 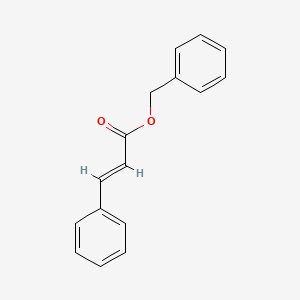 |
0.569 | D01ZJK |  |
0.509 | ||
| ENC001737 | 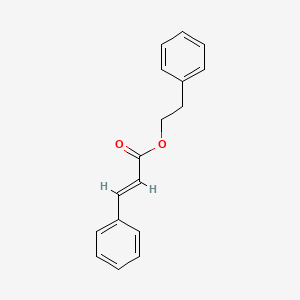 |
0.544 | D0Y0JH | 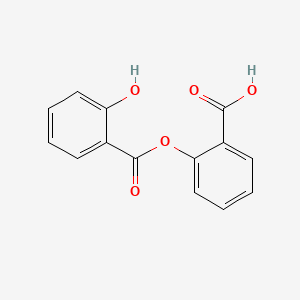 |
0.478 | ||
| ENC001091 | 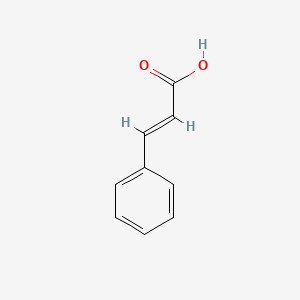 |
0.509 | D0G1VX |  |
0.412 | ||
| ENC000801 | 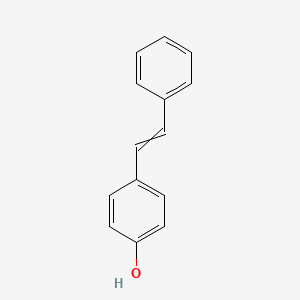 |
0.500 | D07HBX |  |
0.407 | ||
| ENC000093 |  |
0.452 | D00HPK | 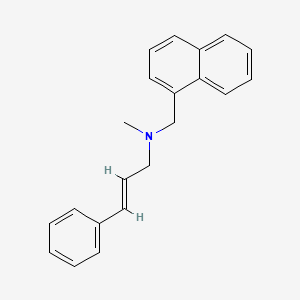 |
0.407 | ||
| ENC000302 | 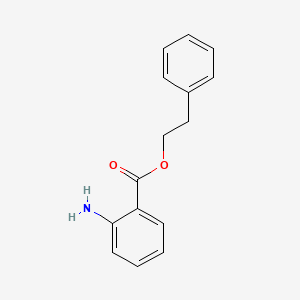 |
0.443 | D0L5PO | 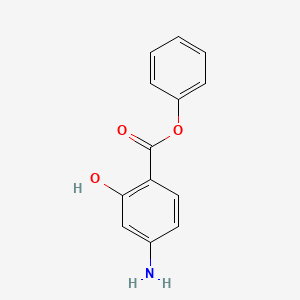 |
0.406 | ||
| ENC000295 | 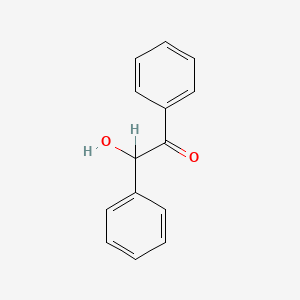 |
0.439 | D0B1FE | 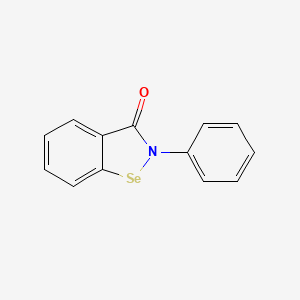 |
0.391 | ||
| ENC001456 | 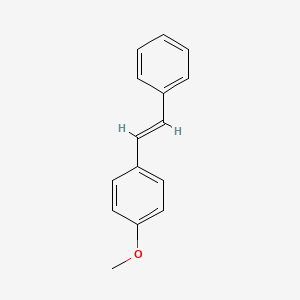 |
0.433 | D04DXN |  |
0.384 | ||
| ENC001805 |  |
0.431 | D08FTG |  |
0.380 | ||
| ENC001443 |  |
0.422 | D0F5ZM | 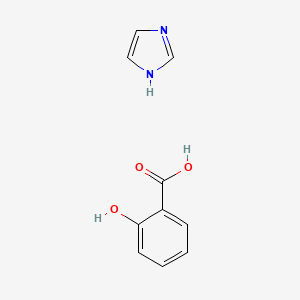 |
0.379 | ||