| Synonyms |
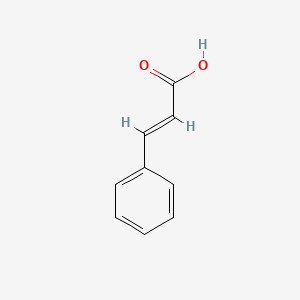
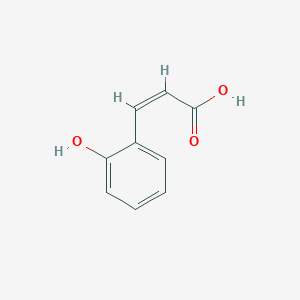
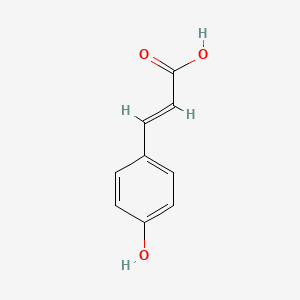
CINNAMIC ACID; TRANS-CINNAMIC ACID; 140-10-3; 621-82-9; 3-Phenylacrylic acid; (E)-Cinnamic acid; trans-3-Phenylacrylic acid; E-Cinnamic Acid; Phenylacrylic acid; (E)-3-phenylprop-2-enoic acid; Zimtsaeure; (2E)-3-phenylprop-2-enoic acid; trans-Cinnamate; 3-Phenylpropenoic acid; Cinnamic acid, (E)-; trans-beta-Carboxystyrene; 3-phenylprop-2-enoic acid; 2-Propenoic acid, 3-phenyl-, (2E)-; Cinnamylic acid; (E)-cinnamate; Benzeneacrylic acid; (E)-3-Phenyl-2-propenoic acid; trans-3-Phenyl-2-propenoic acid; 3-Phenyl-2-propenoic acid; (2E)-3-Phenyl-2-propenoic acid; 2-Propenoic acid, 3-phenyl-, (E)-; t-Cinnamic acid; Benzenepropenoic acid; MFCD00004369; beta-Phenylacrylic acid; PHENYLETHYLENECARBOXYLIC ACID; Cinnamic acid(only trans); Benzylideneacetic acid; (2E)-3-phenylacrylic acid; CHEMBL27246; CHEBI:35697; (2E)-2-Phenyl-2-propenoic acid; U14A832J8D; NSC-9189; FEMA No. 2288; NSC-44010; NSC-623441; NCGC00165979-01; DSSTox_CID_2489; DSSTox_RID_76603; DSSTox_GSID_22489; 28934-71-6; CAS-140-10-3; CCRIS 3190; .beta.-Phenylacrylic acid; EINECS 205-398-1; NSC 44010; (E)-3-Phenylacrylic acid; BRN 1905952; UNII-U14A832J8D; AI3-23709; trans-Zimtsaeure; trans cinnamic acid; 5-Thiazolamine?HCl; b-Phenylacrylic acid; Cinnamic acid, E-; Cinnamic Acid Natural; CINNAMIC ACIDUM; trans-b-Carboxystyrene; Cinnamicacid(onlytrans); PhCH=CHCO2H; trans-3-Phenylacrylate; (E)-3-Phenylacrylate; E-3-phenylpropenoic acid; Trans-Cinnamic Acid,(S); bmse000124; CINNAMIC ACID [MI]; SCHEMBL1332; trans-.beta.-Carboxystyrene; trans-Cinnamic acid, 97%; trans-Cinnamic acid, 99%; WLN: QV1U1R; (E)-3-phenyl-acrylic acid; 3-phenyl-2E-propenoic acid; CINNAMIC ACID [FCC]; Zimtsaeure | trans-Cinnamate; (E)-3-phenylprop-2-enoate; CINNAMIC ACID [FHFI]; CINNAMIC ACID [INCI]; trans-3-Phenyl-2-propenoate; 4-09-00-02002 (Beilstein Handbook Reference); BIDD:ER0586; CINNAMIC ACID [MART.]; tert-.beta.-Phenylacrylic acid; trans-Cinnamic acid, >=99%; (2E)-2-Phenyl-2-propenoate; (2E)-3-Phenyl-2-propenoate; GTPL3203; CINNAMIC ACID [USP-RS]; CINNAMIC ACID [WHO-DD]; DTXSID5022489; BDBM16430; CHEBI:27386; HY-N0610A; NSC9189; NSC44010; STR00363; trans-Cinnamic acid, >=99%, FG; Tox21_112279; Tox21_302137; BBL036895; NSC623441; s3677; STK286093; ZINC16051516; AKOS000118871; CCG-214473; CS-W020005; trans-Cinnamic acid, analytical standard; NCGC00165979-04; NCGC00165979-06; NCGC00255114-01; AC-34658; AS-75479; BP-20203; DB-003797; EN300-19599; trans-Cinnamic acid, purum, >=99.0% (T); A14569; C00423; D70605; EN300-306004; AB00374254-03; trans-cinnamic acid (trans-3-phenylacrylic acid); trans-Cinnamic Acid [Matrix for MALDI-TOF/MS]; A833631; Q164785; SR-05000002380; trans-Cinnamic acid, natural, >=99%, FCC, FG; Q-100150; SR-05000002380-1; W-105037; F2191-0134; trans-Cinnamic Acid Zone Refined (number of passes:40); trans-Cinnamic acid; Phenylacrylic acid;Cinnamylic acid; Z104474406; 1BE36587-A165-4142-9340-18FFE3E03426; CINNAMIC ACID (CONSTITUENT OF CINNAMOMUM VERUM BARK) [DSC]; Cinnamic acid, United States Pharmacopeia (USP) Reference Standard; TRANS-CINNAMIC ACID (CONSTITUENT OF CINNAMOMUM CASSIA BARK) [DSC]
|