NPs Basic Information
|
Name |
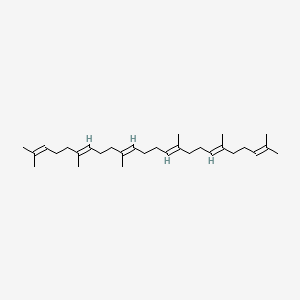
Squalene
|
| Molecular Formula | C30H50 | |
| IUPAC Name* |
(6E,10E,14E,18E)-2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene
|
|
| SMILES |
CC(=CCC/C(=C/CC/C(=C/CC/C=C(/CC/C=C(/CCC=C(C)C)\C)\C)/C)/C)C
|
|
| InChI |
InChI=1S/C30H50/c1-25(2)15-11-19-29(7)23-13-21-27(5)17-9-10-18-28(6)22-14-24-30(8)20-12-16-26(3)4/h15-18,23-24H,9-14,19-22H2,1-8H3/b27-17+,28-18+,29-23+,30-24+
|
|
| InChIKey |
YYGNTYWPHWGJRM-AAJYLUCBSA-N
|
|
| Synonyms |
squalene; 111-02-4; Spinacene; trans-Squalene; Supraene; All-trans-Squalene; 2,6,10,15,19,23-Hexamethyltetracosa-2,6,10,14,18,22-hexaene; Nikko Squalane EX; 7683-64-9; Spinacen; Super Squalene; (E,E,E,E)-Squalene; (6E,10E,14E,18E)-2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene; 2,6,10,15,19,23-Hexamethyl-2,6,10,14,18,22-tetracosahexaene; 11051-27-7; (E)-Squalene; MF59; 2,6,10,14,18,22-Tetracosahexaene, 2,6,10,15,19,23-hexamethyl-; (All-E)-2,6,10,15,19,23-hexamethyl-2,6,10,14,18,22-tetracosahexaene; 7QWM220FJH; 2,6,10,14,18,22-Tetracosahexaene, 2,6,10,15,19,23-hexamethyl-, (all-E)-; CHEBI:15440; Squalene 100 microg/mL in Hexane; NCGC00181323-01; DSSTox_CID_6044; 2,6,10,14,18,22-tetracosahexaene, 2,6,10,15,19,23-hexamethyl-, (6E,10E,14E,18E)-; DSSTox_RID_77993; DSSTox_GSID_26044; Squalen; CAS-111-02-4; EINECS 203-826-1; UNII-7QWM220FJH; squalcnc; Skvalen; CCRIS 711; AddaVax; NCGC00181163-01; MFCD00008912; Squalene, all-trans-; starbld0009755; SQUALENE [INCI]; SQUALENE [MI]; SQUALENE [WHO-DD]; EC 203-826-1; SQUALENE [GREEN BOOK]; Squalene, Spinacene, Supraene; CHEMBL458402; GTPL3054; QSPL 049; MF59 COMPONENT SQUALENE; SQUALENE [EP MONOGRAPH]; DTXSID0026044; HSDB 8242; HY-N1214; NSC93748; ZINC6845904; Tox21_112789; Tox21_113239; NSC-93748; s4862; AKOS015917344; CCG-268779; DB11460; 2,6,10,15,19,23-Hexamethyl-2,6,10,14,18,22-tetracosahexaene, (all-E)-; 2,6,10,14,18,22-Tetracosahexaene, 2,6,10,15,19,23-hexamethyl-, (2E,6E,10E,14E,18E)-; AS-56045; BS-49251; CS-0016601; H0097; MONTANIDE ISA 720 COMPONENT SQUALENE; C00751; E80634; Q407560; SR-01000944749; J-002505; SR-01000944749-1; 3704365A-1DAB-4AAA-A0D0-CAE7A96723AC; (all-E)-2,6,10,15,19,23-Hexamethyl-2,6,10,14,18,22-tetraco-sahexaene; 2,6,10,15,19,23-Hexamethyltetracosa-(2E,6E,10E,14E,18E,22E)-2,6,10,14,18,22-hexaene
|
|
| CAS | 111-02-4 | |
| PubChem CID | 638072 | |
| ChEMBL ID | CHEMBL458402 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 410.7 | ALogp: | 11.6 |
| HBD: | 0 | HBA: | 0 |
| Rotatable Bonds: | 15 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 0.0 | Aromatic Rings: | 0 |
| Heavy Atoms: | 30 | QED Weighted: | 0.152 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.912 | MDCK Permeability: | 0.00000935 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.023 |
| Human Intestinal Absorption (HIA): | 0.548 | 20% Bioavailability (F20%): | 0.86 |
| 30% Bioavailability (F30%): | 0.976 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.115 | Plasma Protein Binding (PPB): | 95.56% |
| Volume Distribution (VD): | 4.617 | Fu: | 2.99% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.172 | CYP1A2-substrate: | 0.157 |
| CYP2C19-inhibitor: | 0.18 | CYP2C19-substrate: | 0.068 |
| CYP2C9-inhibitor: | 0.326 | CYP2C9-substrate: | 0.974 |
| CYP2D6-inhibitor: | 0.278 | CYP2D6-substrate: | 0.092 |
| CYP3A4-inhibitor: | 0.543 | CYP3A4-substrate: | 0.097 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 4.249 | Half-life (T1/2): | 0.041 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.009 | Human Hepatotoxicity (H-HT): | 0.688 |
| Drug-inuced Liver Injury (DILI): | 0.004 | AMES Toxicity: | 0 |
| Rat Oral Acute Toxicity: | 0 | Maximum Recommended Daily Dose: | 0.529 |
| Skin Sensitization: | 0.983 | Carcinogencity: | 0.007 |
| Eye Corrosion: | 0.312 | Eye Irritation: | 0.885 |
| Respiratory Toxicity: | 0.001 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC001466 |  |
0.529 | D09XWD |  |
0.690 | ||
| ENC001465 |  |
0.529 | D05XQE | 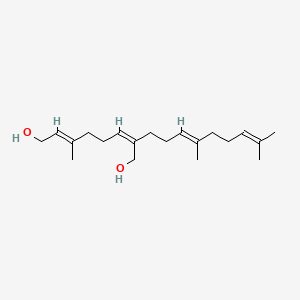 |
0.495 | ||
| ENC001873 | 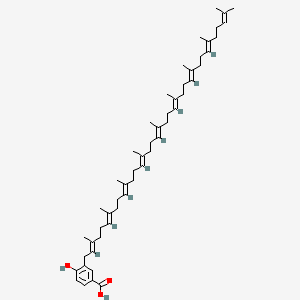 |
0.490 | D01ZUA | 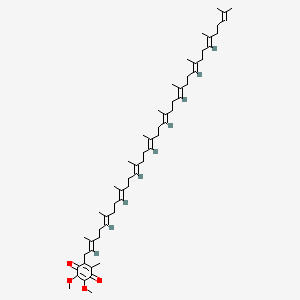 |
0.433 | ||
| ENC001716 | 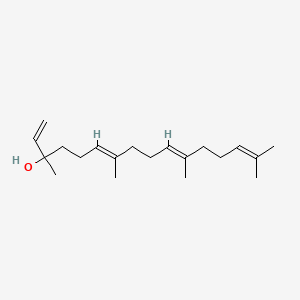 |
0.453 | D03VFL | 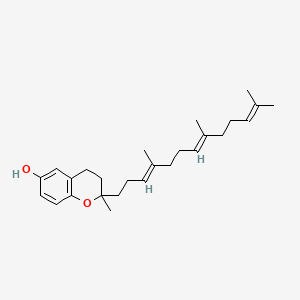 |
0.356 | ||
| ENC001464 | 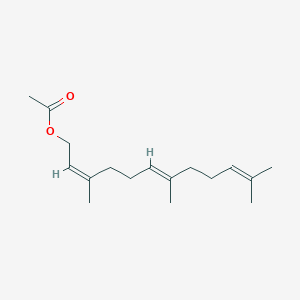 |
0.446 | D0UE9X | 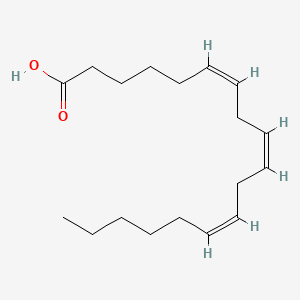 |
0.178 | ||
| ENC001096 | 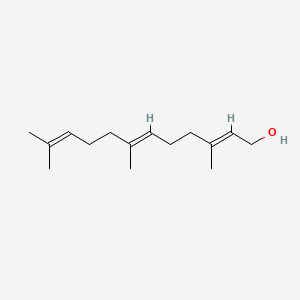 |
0.437 | D0O1TC |  |
0.169 | ||
| ENC001462 |  |
0.437 | D06BLQ |  |
0.168 | ||
| ENC001717 |  |
0.404 | D0M1PQ |  |
0.161 | ||
| ENC002413 |  |
0.404 | D00FSV |  |
0.153 | ||
| ENC001467 |  |
0.368 | D0X7XG |  |
0.153 | ||