NPs Basic Information
|
Name |
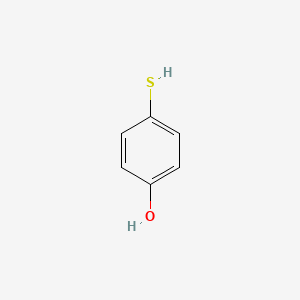
4-Mercaptophenol
|
| Molecular Formula | C6H6OS | |
| IUPAC Name* |
4-sulfanylphenol
|
|
| SMILES |
C1=CC(=CC=C1O)S
|
|
| InChI |
InChI=1S/C6H6OS/c7-5-1-3-6(8)4-2-5/h1-4,7-8H
|
|
| InChIKey |
BXAVKNRWVKUTLY-UHFFFAOYSA-N
|
|
| Synonyms |
4-Mercaptophenol; 4-Hydroxythiophenol; 637-89-8; 4-Sulfanylphenol; 4-Hydroxybenzenethiol; Phenol, 4-mercapto-; p-Mercaptophenol; p-Hydroxythiophenol; Monothiohydroquinone; Thiohydroquinone; Phenol, p-mercapto-; 4-Hydroxy Thiophenol; Hydroquinone, monothio-; USAF B-57; p-hydroxybenzenethiol; NSC 46192; TAL4TS4AC3; NSC-46192; 4-mercapto-phenol; EINECS 211-307-6; MFCD00004850; BRN 2039306; AI3-32249; 4-mercapto phenol; p-hydroxy thiophenol; 4-hydroxy-thiophenol; 4-Sulfanylphenol #; para-hydroxy thiophenol; UNII-TAL4TS4AC3; 4-Mercaptophenol, 97%; WLN: L6V DYJ DUS; SCHEMBL62871; DTXSID2073223; BXAVKNRWVKUTLY-UHFFFAOYSA-; NSC46192; BBL100598; STL554392; ZINC19735431; AKOS005254768; PS-5184; 4-Mercaptophenol, technical grade, 90%; AC-10071; DB-054547; FT-0618753; H0662; EN300-44305; S10242; 4-Mercaptophenol, Vetec(TM) reagent grade, 89%; A934091; J-512663; W-104875; F0001-1734
|
|
| CAS | 637-89-8 | |
| PubChem CID | 240147 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 126.18 | ALogp: | 1.1 |
| HBD: | 2 | HBA: | 2 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 21.2 | Aromatic Rings: | 1 |
| Heavy Atoms: | 8 | QED Weighted: | 0.511 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.462 | MDCK Permeability: | 0.00000935 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.024 | 20% Bioavailability (F20%): | 0.984 |
| 30% Bioavailability (F30%): | 0.983 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.16 | Plasma Protein Binding (PPB): | 70.64% |
| Volume Distribution (VD): | 3.103 | Fu: | 26.16% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.805 | CYP1A2-substrate: | 0.395 |
| CYP2C19-inhibitor: | 0.394 | CYP2C19-substrate: | 0.231 |
| CYP2C9-inhibitor: | 0.084 | CYP2C9-substrate: | 0.807 |
| CYP2D6-inhibitor: | 0.217 | CYP2D6-substrate: | 0.83 |
| CYP3A4-inhibitor: | 0.051 | CYP3A4-substrate: | 0.28 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 15.447 | Half-life (T1/2): | 0.865 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.003 | Human Hepatotoxicity (H-HT): | 0.039 |
| Drug-inuced Liver Injury (DILI): | 0.425 | AMES Toxicity: | 0.614 |
| Rat Oral Acute Toxicity: | 0.824 | Maximum Recommended Daily Dose: | 0.102 |
| Skin Sensitization: | 0.893 | Carcinogencity: | 0.778 |
| Eye Corrosion: | 0.96 | Eye Irritation: | 0.992 |
| Respiratory Toxicity: | 0.605 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000086 |  |
0.571 | D03UOT |  |
0.571 | ||
| ENC000005 |  |
0.516 | D0U5QK |  |
0.444 | ||
| ENC000318 | 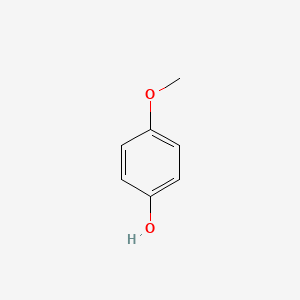 |
0.516 | D0W1RY | 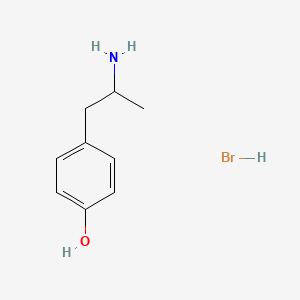 |
0.432 | ||
| ENC000007 | 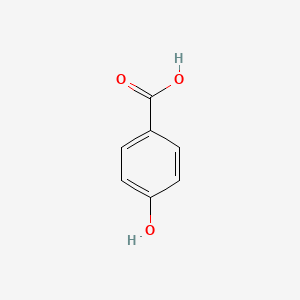 |
0.485 | D01CRB | 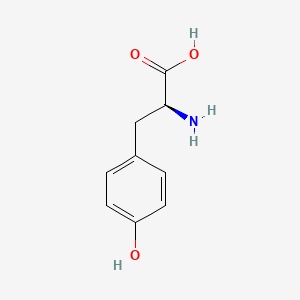 |
0.390 | ||
| ENC000665 | 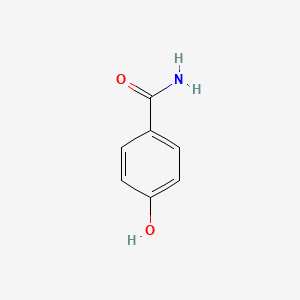 |
0.485 | D02WAB | 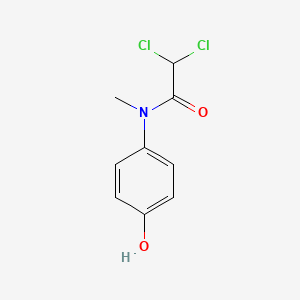 |
0.372 | ||
| ENC000200 | 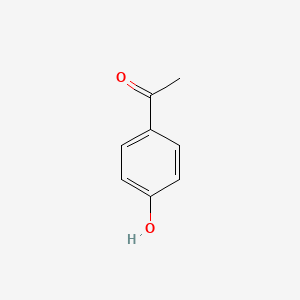 |
0.485 | D0B3QM | 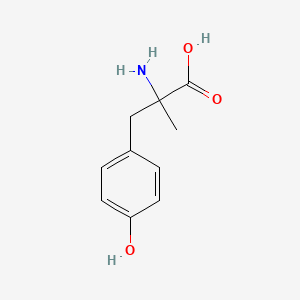 |
0.372 | ||
| ENC000740 | 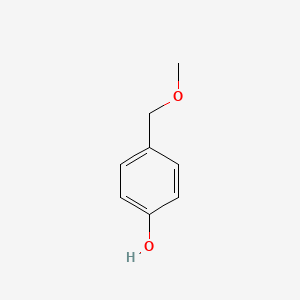 |
0.471 | D0S2BV | 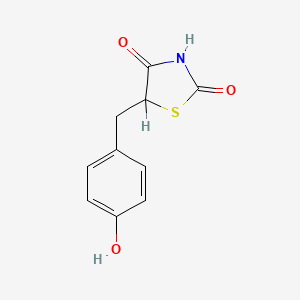 |
0.333 | ||
| ENC000350 | 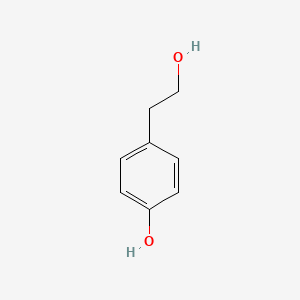 |
0.471 | D0H6TP | 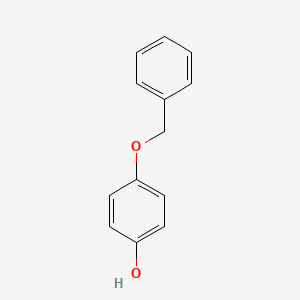 |
0.320 | ||
| ENC000676 | 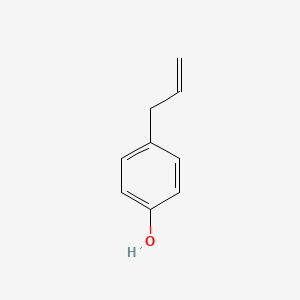 |
0.471 | D0O3FG | 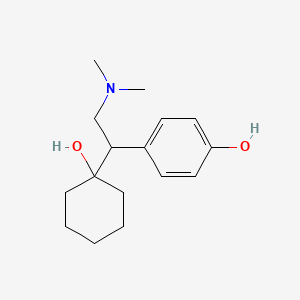 |
0.271 | ||
| ENC000195 | 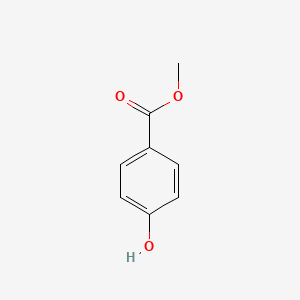 |
0.444 | D0Y2NE | 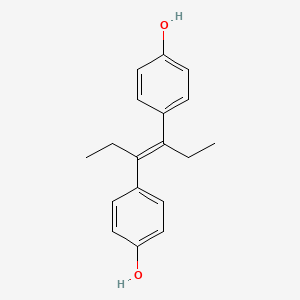 |
0.258 | ||