NPs Basic Information
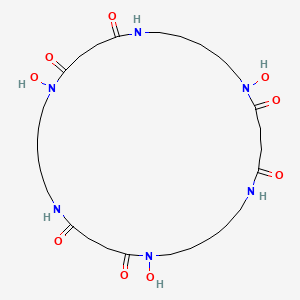
|
Name |
Nocardamine
|
| Molecular Formula | C27H48N6O9 | |
| IUPAC Name* |
1,12,23-trihydroxy-1,6,12,17,23,28-hexazacyclotritriacontane-2,5,13,16,24,27-hexone
|
|
| SMILES |
C1CCNC(=O)CCC(=O)N(CCCCCNC(=O)CCC(=O)N(CCCCCNC(=O)CCC(=O)N(CC1)O)O)O
|
|
| InChI |
InChI=1S/C27H48N6O9/c34-22-10-14-26(38)32(41)20-8-3-6-18-30-24(36)12-15-27(39)33(42)21-9-2-5-17-29-23(35)11-13-25(37)31(40)19-7-1-4-16-28-22/h40-42H,1-21H2,(H,28,34)(H,29,35)(H,30,36)
|
|
| InChIKey |
NHKCCADZVLTPPO-UHFFFAOYSA-N
|
|
| Synonyms |
Nocardamine; desferrioxamine E; Nocardamin; 26605-16-3; Deferrioxamine E; 1,12,23-trihydroxy-1,6,12,17,23,28-hexaazacyclotritriacontane-2,5,13,16,24,27-hexone; 1,12,23-trihydroxy-1,6,12,17,23,28-hexazacyclotritriacontane-2,5,13,16,24,27-hexone; 1,6,12,17,23,28-Hexaazacyclotritriacontane-2,5,13,16,24,27-hexone, 1,12,23-trihydroxy-; MLS000876815; CHEBI:50437; SMR000440583; Q645668975; 1,12,23-Trihydroxy-1,6,12,17,23,28-hexaazacyclotritriacontane-2,5,13,16,24,27-hexaone; UNII-Q645668975; PROFERRIOXAMINE E; NOCARDAMIN [MI]; DEFERRIFERRIOXAMINE E; MLS003559977; CHEMBL505734; cid_161532; MEGxm0_000122; SCHEMBL3725976; ACon0_001491; ACon1_001281; BDBM47827; DTXSID00181161; HMS2268B17; HB3998; ZINC17545546; BS-1596; NCGC00180673-01; J-016496; Q27122069; Nocardamine; Norcardamin; Deferrioxamine E; Desferri-ferrioxamin-E; Proferrioxamine-E; 1,12,23-trihydroxy-1,6,12,17,23,28-hexazacyclotritriacontane-2,5,13,16,24,27-triquinone; 1,12,23-tris(oxidanyl)-1,6,12,17,23,28-hexazacyclotritriacontane-2,5,13,16,24,27-hexone
|
|
| CAS | 26605-16-3 | |
| PubChem CID | 161532 | |
| ChEMBL ID | CHEMBL505734 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 600.7 | ALogp: | -2.2 |
| HBD: | 6 | HBA: | 9 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 209.0 | Aromatic Rings: | 1 |
| Heavy Atoms: | 42 | QED Weighted: | 0.221 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.478 | MDCK Permeability: | 0.00003020 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.996 |
| Human Intestinal Absorption (HIA): | 1 | 20% Bioavailability (F20%): | 1 |
| 30% Bioavailability (F30%): | 1 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.031 | Plasma Protein Binding (PPB): | 21.48% |
| Volume Distribution (VD): | 1.416 | Fu: | 66.95% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.001 | CYP1A2-substrate: | 0.056 |
| CYP2C19-inhibitor: | 0.035 | CYP2C19-substrate: | 0.028 |
| CYP2C9-inhibitor: | 0.073 | CYP2C9-substrate: | 0.968 |
| CYP2D6-inhibitor: | 0.047 | CYP2D6-substrate: | 0.045 |
| CYP3A4-inhibitor: | 0.045 | CYP3A4-substrate: | 0.002 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 9.062 | Half-life (T1/2): | 0.113 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.106 | Human Hepatotoxicity (H-HT): | 0.325 |
| Drug-inuced Liver Injury (DILI): | 0.024 | AMES Toxicity: | 0.016 |
| Rat Oral Acute Toxicity: | 0.744 | Maximum Recommended Daily Dose: | 0.166 |
| Skin Sensitization: | 0.168 | Carcinogencity: | 0.001 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.038 |
| Respiratory Toxicity: | 0.001 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC002169 | 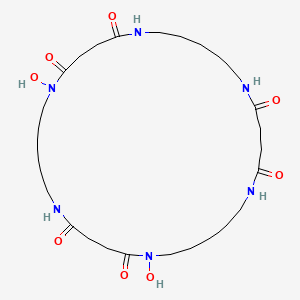 |
0.841 | D0L5RW | 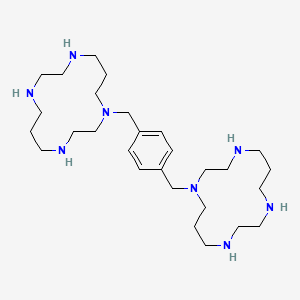 |
0.197 | ||
| ENC001146 | 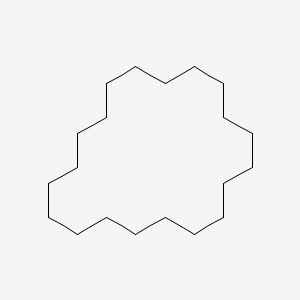 |
0.221 | D04APR | 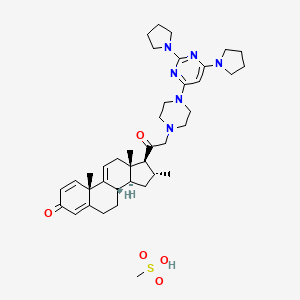 |
0.193 | ||
| ENC001017 | 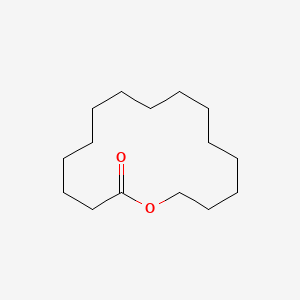 |
0.219 | D0F9GE | 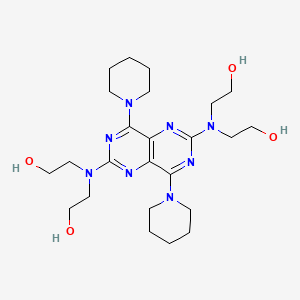 |
0.188 | ||
| ENC001147 | 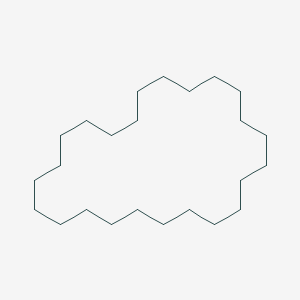 |
0.212 | D00SBN |  |
0.180 | ||
| ENC000840 |  |
0.197 | D0S5NG |  |
0.175 | ||
| ENC004418 |  |
0.195 | D07HOB | 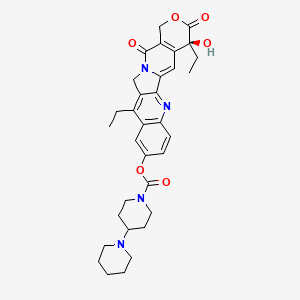 |
0.171 | ||
| ENC005563 | 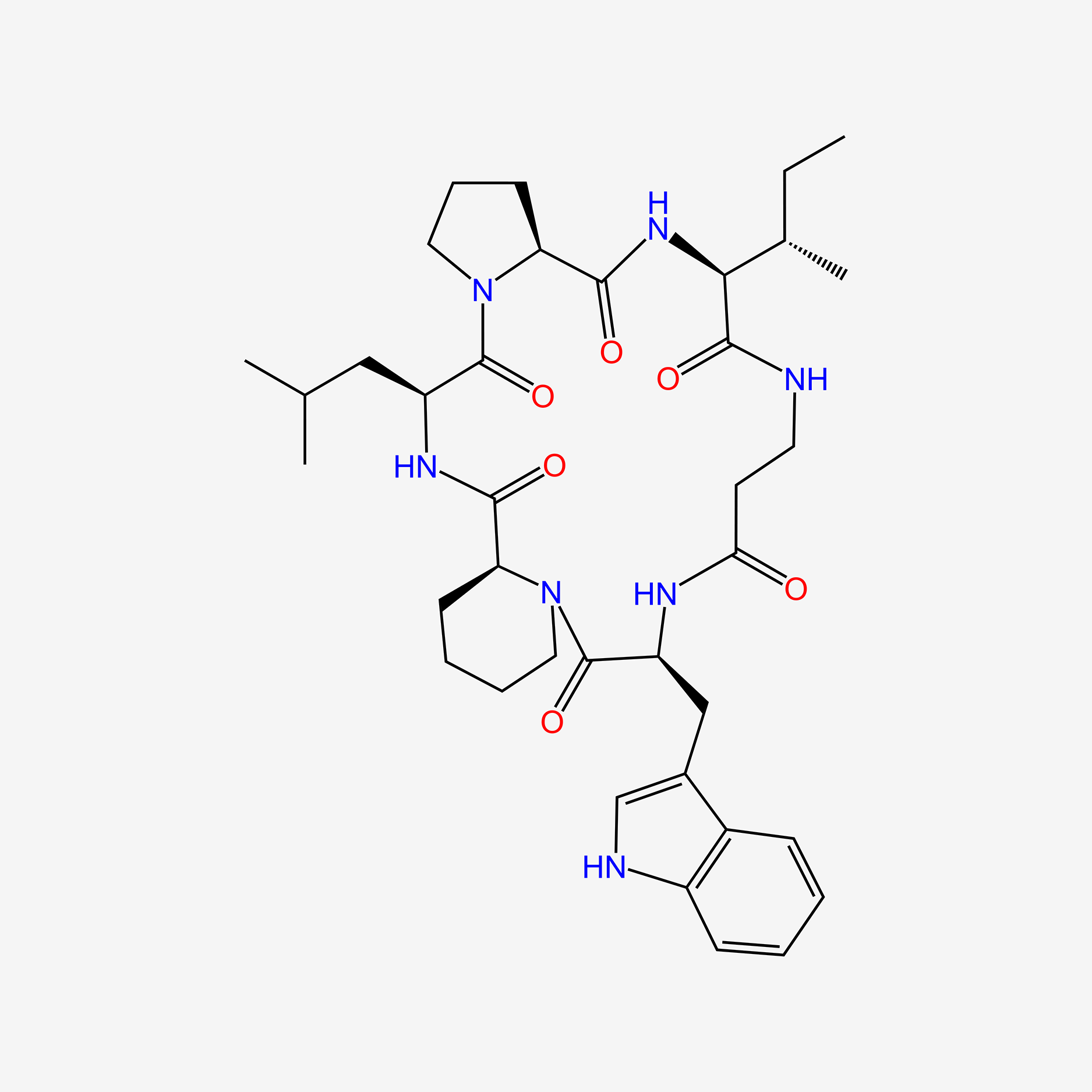 |
0.195 | D0F3IP | 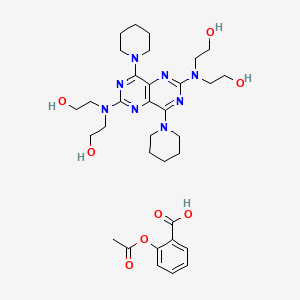 |
0.169 | ||
| ENC001527 | 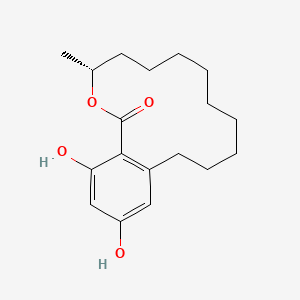 |
0.193 | D0Z8HG | 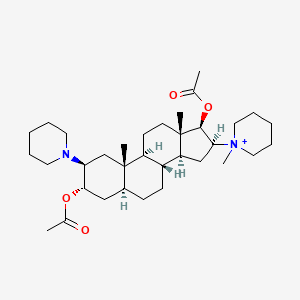 |
0.162 | ||
| ENC000893 | 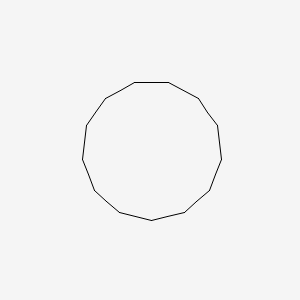 |
0.191 | D09GFL | 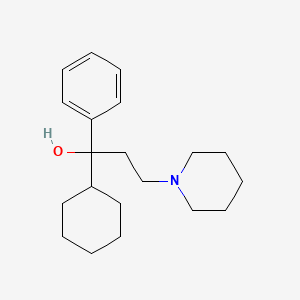 |
0.159 | ||
| ENC005007 | 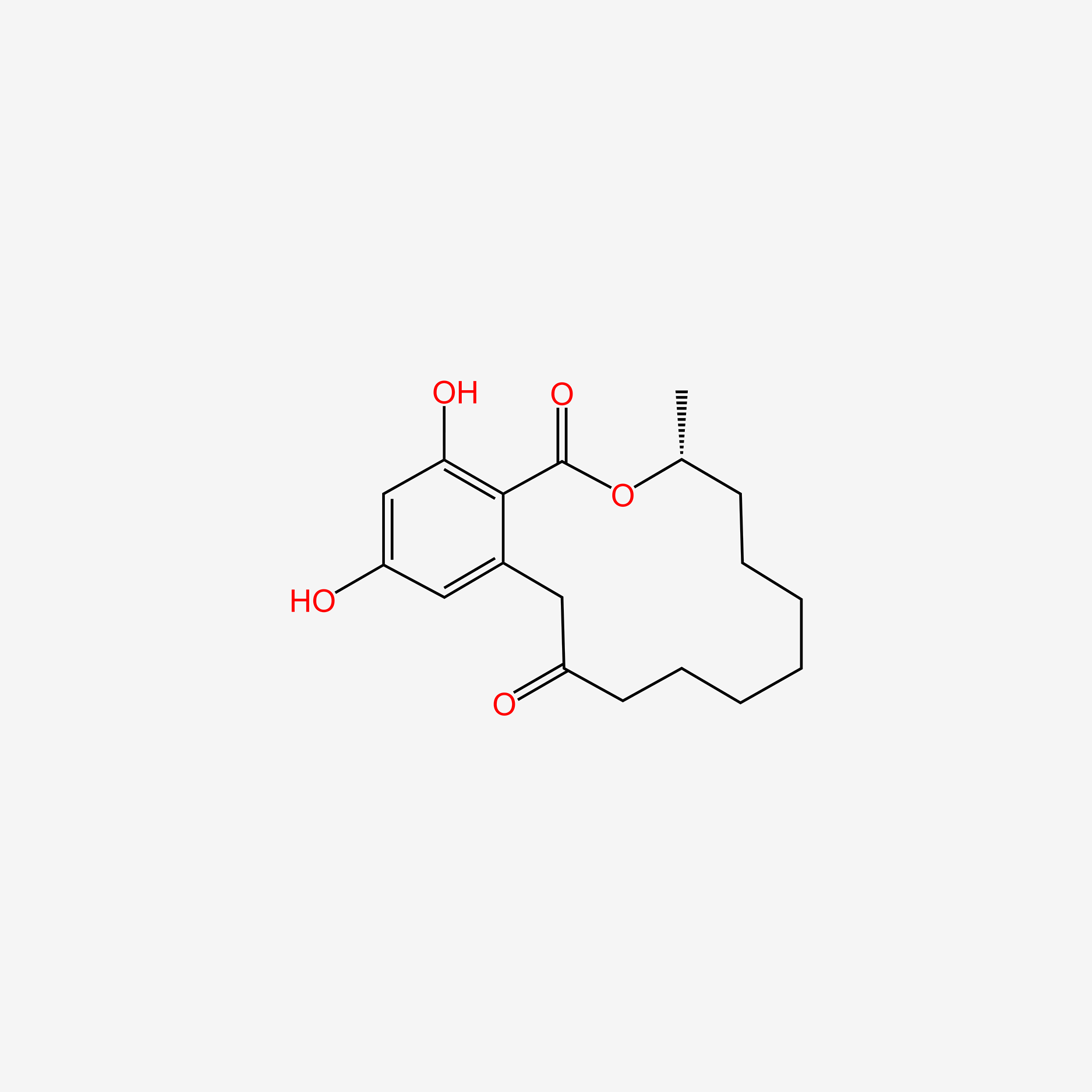 |
0.191 | D07XVN | 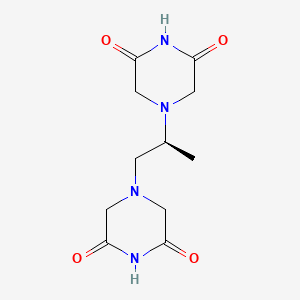 |
0.158 | ||